Novel Green Hydrogel Material Using Bacterial Cellulose
Amorn Chaiyasat1, 2 , Sirinard Jearanai1, Somporn Moonmangmee3, Duangtip Moonmangmee4, Lew P Christopher5, Md Nur Alam5 and Preeyaporn Chaiyasat1,2
, Sirinard Jearanai1, Somporn Moonmangmee3, Duangtip Moonmangmee4, Lew P Christopher5, Md Nur Alam5 and Preeyaporn Chaiyasat1,2
1Department of Chemistry, Faculty of Science and Technology, Rajamangala University of Technology Thanyaburi, Klong6, Thanyaburi, Pathumthani 12110, Thailand.
2Advanced Materials Design and Development (AMDD) Research Unit, Faculty of Science and Technology, Rajamangala University of Technology Thanyaburi, Klong 6, Thanyaburi, Pathumthani 12110, Thailand.
3Biodiversity Research Center, Thailand Institute of Scientific and Technological Research, Pathum Thani 12120, Thailand.
4Department of Microbiology, Faculty of Science, King Mongkut’s University of Technology Thonburi, Bangkok 10140, Thailand.
5Biorefining Research Institute, Lakehead University, 1294 Balmoral Street, Thunder Bay, Ontario, P7B 5Z5, Canada.
Corresponding Author E-mail: a_chaiyasat@mail.rmutt.ac.th
DOI : http://dx.doi.org/10.13005/ojc/340404
Article Received on : 11-05-2018
Article Accepted on : 15-06-2018
Article Published : 25 Jul 2018
The green bacterial cellulose (BC)-based hydrogel materials have successfully prepared by modification and crosslink BC. BC was derived from acetic acid bacteria isolated and selected from ripe fruits. The production of BC was performed by fermentation in various media. It was found that using liquid potato medium represented the highest thickness of BC film (0.80 cm) with 2 wt% solid content covered the media. To reduce the crystallization of BC, carboxyl group was introduced onto BC chains using a carboxymethylation reaction giving carboxymethyl BC (CMBC) and subsequently crosslinked with divinyl sulfone (DVS). The extent of crosslinking influenced on the swelling properties of the hydrogels. Using large DVS amounts (>30 wt%-of CMBC), dense macromolecular network with less capacity spaces in the hydrogel was formed. The maximum water retention value of green hydrogels containing ~3.0 mmol carboxyl groups/g CMBC reached 27 (g/g).
KEYWORDS:Bacterial Cellulose; Biopolymer; Carboxymethyl Bacterial Cellulose; Hydrogel Material
Download this article as:| Copy the following to cite this article: Chaiyasat A, Jearanai S, Moonmangmee S, Moonmangmee D, Christopher L. P, Alam M. N, Chaiyasat P. Novel Green Hydrogel Material Using Bacterial Cellulose. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Chaiyasat A, Jearanai S, Moonmangmee S, Moonmangmee D, Christopher L. P, Alam M. N, Chaiyasat P. Novel Green Hydrogel Material Using Bacterial Cellulose. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47603 |
Introduction
Hydrogels are three-dimensional crosslinked hydrophilic materials to avoid the dissolution of the polymer chains in the medium1 which are able to absorb and retain large amount of liquids, bodily fluids and blood solutions.2,3 Normally, the polymer chains are crosslinked via either physical or chemical crosslinking as Van der Waals interaction or covalent bond, respectively. Due to hydrophilic properties of hydrogel with high sorption capacity, they have been extensively used in various applications such as personal care products,4,5 agriculture,6,7 foods,8,9 and biomedical applications.3,10 Usually, polymer-based hydrogel materials are polyelectrolytes such as crosslinked poly(sodium acrylate), polyacrylamide and their copolymers.4,7,11,12 They are produced by either solution polymerization of partially neutralized acrylic acid or suspension polymerization to form a gel. However, polyelectrolytes are currently derived from petroleum products. Because of environmental issues, natural polymer-based hydrogels as renewable or biologically degradable polymers with lower toxicity than most synthetic polymers are currently interesting.13,14 Most of cellulose-based hydrogel currently used are carboxyalkyl cellulose, gum, carboxyalkyl starch, cellulose sulphate, etc.7,13 containing synthetic polymers, such as polyacrylates, sulfonated polystyrene, polyvinyl alcohol and etc. To increase water absorptivity, cellulose is normally crosslinked with various crosslinkers such as succinic anhydride through etherification reaction15 and divinyl sulfone (DVS).2,16 To the best of our knowledge, there is a few research of cellulose-based hydrogel production with high purity bacteria cellulose (BC). Therefore, in this work, novel green hydrogel material using BC will be presented. The process is based on a chemical modification method for carboxymethylation of BC to generate carboxyl groups prior to crosslinked with DVS. The new preparation process, the properties of the obtained new hydrogel material and its potential application will be reported.
Experimental Materials
Reagent grade sodium chloroacetate, hydroxyethyl cellulose (HEC), DVS were purchased from Sigma-Aldrich (Mississauga, Ontario, Canada). Sodium hydroxide (NaOH), potassium hydroxide (KOH), propanol and ethanol were supplied by Thermo Fisher Scientific (Whitby, Ontario, Canada). All chemicals were used as received.
Preparation of Bacterial Cellulose (BC)
Bacterial cellulose (BC) was microbially produced by an acetic acid bacterium, Acetobactor xylinum which isolated from ripe pineapple. The isolation and purification of A. xylinum was done by cross streak method. The bacteria were streak for several times until obtaining the single colony having clear zone onto a solid media (potato agar medium) which contains (%w/v): potato extract, peptone 1.0, yeast extract 1.0, D-glucose 0.5, glycerol 1.0, agar 1.5-1.7 and calcium carbonate 0.5. The selected colony of A. xylinum was then inoculated into 5 ml of potato medium broth without CaCO3 at room temperature for 1-2 days in order to prepare the starter culture. Thereafter, the starter culture was then poured into various kinds of modified culture broths (1 l) supplemented either with coconut, pineapple, or pineapple containing 1 wt% of glucose and potato medium which contain different carbon/nitrogen (C/N) ratios (carbon and nitrogen sources are glucose, and yeast extract and peptone, respectively). The culture media were incubated statically at room temperature for 7 days. Finally, the BC film was formed on the top of each media. Thereafter, BC was purified by soaking in acetone for 2 h in triplicate before dried at room temperature. The dried BC was cut to be the small pieces (d = 1.0 mm).
Table 1: Reagent amount of the hydrogel preparation from CMBC using DVS as a crosslinker
|
Run |
Ingredient |
||||
|
CMBCa (g) |
CMCb (g) |
HEC (g) |
DVS (g) |
Water (g) |
|
|
1 |
0.40 |
– |
0.15 |
2.00 |
47.45 |
|
2 |
0.75 |
– |
0.25 |
2.00 |
47.00 |
|
3 |
2.25 |
– |
0.75 |
2.00 |
45.00 |
|
4 |
– |
2.25 |
0.75 |
2.00 |
45.00 |
|
5 |
0.75 |
– |
0.25 |
0.15 |
48.85 |
|
6 |
0.75 |
– |
0.25 |
0.30 |
48.70 |
|
7 |
0.75 |
– |
0.25 |
0.65 |
48.35 |
a obtained from bacteria; b obtained from wood
Preparation of Carboxymethylated Bacterial Cellulose (CMBC)
Carboxymethylation of BC was carried out according to a previously described method17 with some modifications. BC swollen with sodium hydroxide was firstly prepared by adding BC (5 wt% in final solution) into the mixture solution of sodium hydroxide: propanol: water (4:86:11 wt% ratio) at room temperature for 1 h. Subsequently, sodium chloroacetic solution (50 wt%) was added to BC slurry for fourth of the slurry in a period of 30 min. Thereafter, the mixture temperature was increased to 50°C for 4 h before stopping the reaction by the addition of 50 ml of anhydrous ethanol. The obtained CMBC was then filtered through a nylon cloth and dispersed in a methanol solution (70 wt%) with mild stirring rate. For the carboxyl group determination, the obtained CMBC solution was added by an aqueous solution of 0.5 M HCl until the pH of the solution mixture was below 3 where CMBC salt was converted to the acid form (H-CMBC). To remove the excess acid and others impurities, the precipitated H-CMBC after filtration was washed with 70% ethanol for 3 times and then washed with anhydrous ethanol before dried.
Preparation of Hydrogels from CMBC Using DVS Crosslinker
CMBC and hydroxyethyl cellulose (HEC) were firstly mixed in the ratio of 3:1 at various total concentrations (1-6 wt%) excluding crosslinker. Thereafter, the cellulose solution was added by NaOH to obtain the alkaline solution at pH of 12.5 where the final concentration of NaOH was 0.02 M. The hydrogel was finally obtained by adding DVS (4 wt%) at 0°C. The reagent amount of the hydrogel preparation using DVS as a crosslinker was shown in Table 1.
Characterizations
The chemical structure of CMBC was analyzed by Fourier Transform Infrared (FT-IR) spectrometer (Bruker Tensor 37, Bruker, Ettlingen, Germany) with PIKE miracle diamond attenuated total reflectance (ATR) accessory. The dried samples were placed directly on the ATR crystal. The carboxyl content of the CMBC was determined using a back titration method17 with a METER pH/conductivity S470-KIT (Mettler-Toledo GmbH, Greifensee, Switzerland) titrator. A certain amount of 0.1 g of H-CMBC sample was dispersed in 30 g water with mild stirring rate until a well-disperse solution was formed. The pH of the dispersion was then adjusted to 3.5 with the addition of 0.1 M HCl. After the known amount (5 ml) of 0.1 M NaOH solution was added to the mixture, the excess NaOH was then back-titrated with standard HCl solution (0.01 M) using phenolphthalein as an indicator. The titration was repeated three times. The carboxyl content of CMBC was calculated by the following equation (Eq. 1):
[COOH] = (VNaOH x MNaOH – VHCl x MHCl)/DW (1)
where [COOH] is carboxyl content of crosslinked gels (mmol/g-CMBC), VNaOH and MNaOH are the volume (ml) and concentration of the adding NaOH (mmol/ml), respectively, and DW is dried weight (g) of CMBC. Water Retention Value (WRV) of hydrogel was measured as a follow. The hydrogels were immersed in distilled water or 0.9 wt% NaCl aqueous (saline) solution. At each interval time, the swelling hydrogels were withdrawn from the mixture and weighed out after removing the excess liquid off the gel surface. WRV, a measure of the dynamic water absorption properties of the gel, was calculated (Eq. 2) as follows:
WRV = (Wt – Wd)/Wd × 100 (2)
Wt is weight of wet gel at time t, and Wd is weight of the hydrogel in dried state.
Results and Discussion
The BC was grown up covering the mediums with various C/N ratios. It was found that the thickness of the obtained BC were 0.4 cm, 0.4 cm, 0.4 cm, 0.45 cm and 0.80 cm for coconut, pineapple, pineapple with 1 wt% of glucose and potato mediums, respectively. Based on the highest thickness of BC, potato medium was then used as the optimum medium. In addition, the obtained BC film (as shown in the Fig. 1) represented high water retention as only 2 wt% of dried BC with 98 wt% of water in the obtained BC film. It is well known that basic structure of BC consists of β-1→4 glucan chains where they connected the other chains by both inter-and intra-hydrogen bonding18 with high surface area and porosity.19,20 Therefore, BC can absorb large amount of water. However, after drying, the BC cannot re-absorb the water. To use BC as the hydrogel, the BC then needed to be modified.
 |
Figure 1: The BC film formed on potato medium |
To reduce hydrogen bonding strength connected between cellulose chains of BC, carboxyl group was introduced via carboxymethylation using chloroacetic acid. Due to NaOH reacts with some hydroxyl groups of BC and generates a strong nucleophile, most of the alkoxide ion in BC chains attacks the chloroacetate to obtain CMBC.21 The mechanism of CMBC preparation was shown in Fig. 2. FTIR spectra of BC and CMBC were shown in Fig. 3. In the case of BC, the characteristic absorption peaks at ~3,300 cm-1 and ~2,900 cm-1 corresponded to the stretching vibration of OH group and C–H stretching vibration, respectively. All important absorption peaks presented in BC were obtained in the case of CMBC. However, much broad peak at 3,000-3,600 cm-1 was obtained in the case of CMBC which corresponded to OH stretching of carboxyl group. In addition, about 1,700 cm-1 corresponding to C=O stretching of acid (carboxyl group) could be observed only in CMBC. This indicated that carboxyl group was successfully introduced to BC chains.
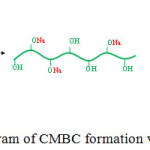 |
Figure 2: Schematic diagram of CMBC formation via carboxymethylation. |
The amount of introduced carboxyl group into the cellulose chains could be obtained by a back titration. Before the measurement, the pH of the aqueous medium containing CMBC chains was adjusted to 3.5 to ensure that all carboxylate salts was changed to be carboxyl groups. Based on the equation 1, 2.50-3.50 mmol/g-CMBC of carboxyl group in modified bacteria cellulose chain were obtained.
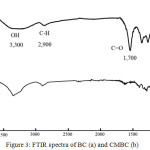 |
Figure 3: FTIR spectra of BC (a) and CMBC (b) |
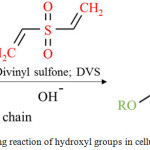 |
Figure 4: Crosslinking reaction of hydroxyl groups in cellulose chain with DVS |
To obtain the hydrogel having stable structure and effective water swelling, CMBC chains were linked as 3D hydrophilic network via covalent bond using a commonly crosslinker as a DVS. DVS can connect both chains via hydroxyl group of cellulose as shown in Fig. 4. It is well known that CMBC normally represents high crytallization structure. It seemed difficult to form intermolecular crossilnking. However, the solution of CMBC containing HEC could reduce intramolecular crosslinking and proceeded 3D hydrophilic network structure.2,22 In this work, the ratio of CMBC: HEC was fixed at 3:1 with the concentration range of total cellulose of 1-6 wt% where the DVS concentration was used at 4 wt% for all conditions (Run1-3). It was found that at 1 wt% of cellulose the hydrogel structure could not formed. It may be due to low CMBC content where it could not absorb any water. The WRV increased with the cellulose content as 3.6 and 8.7 g/g-hydrogel, respectively, for 2 (Run 2) and 6 wt% (Run 3). However, their WRV seems far from that (10.7 g/g-hydrogel) of hydrogel produced from wood (Run 4). After water absorption, the hydrogel obtained from CMC represented soft gel whereas quite hard gels were obtained from CMBC. It may be due to higher crytallization of CBMC than that of CMC. In addition, the obtained hydrogel from 2 wt% of CMBC was softer than that from 6 wt% and similar to the hydrogel produced from CMC. The low WRV of hydrogel produced from 2 wt% CMBC may be due to high crosslink density (200 wt% of total cellulose). Therefore, the crosslinker content was decreased as 15 (Run 5), 30 (Run 6) and 65 (Run 7) wt% of total cellulose. The water swollen CMBC hydrogel with various crosslink contents were represented in Fig. 5. It was found that the WRV increased with crosslinker content as 24.1 and 26.6 g/g-hydrogel for 15 and 30 wt% of DVS. However, negative result was obtained with the other increases of crosslinker content as given 18.0 (Run 7) and 3.6 (Run 2) g/g-hydrogel for 65 and 200 wt% of DVS, respectively.
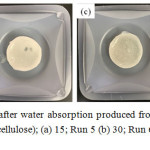 |
Figure 5: Hydrogel photos after water absorption produced from CMBC crosslinked with various DVS (wt% of total cellulose); (a) 15; Run 5 (b) 30; Run 6 (c) 65; Run 7 and (d) 200; Run 2 |
Conclusion
Green hydrogel from bacteria cellulose was successfully prepared in the first time. The total solid content of cellulose affected on the water retention value. In addition, WRV significantly depended on the amount of DVS. Based on high purity and highly-absorbent of the obtained hydrogel, it might find the new uses in high-value hygiene, food, agricultural and pharmaceutical products.
Acknowledgements
This work was supported by RMUTT annual government statement of expenditure in 2017 (NRMS No. 2560A16502026 given to A.C. and P.C) and by the Biorefining Research Institute (BRI) at Lakehead University. Special thanks to Mr. Michael Sorokopud at Lakehead University Instrumentation Laboratory for the FTIR and SEM facility.
References
- Bakass, M.; Mokhlisse, A. and Lallemant, M. Journal of Applied Polymer Science 2002, 83(2), 234-243.
CrossRef - Astrini, N.; Anah, L. and Haryono, A. Procedia Chemistry 2012, 4, 275-281.
CrossRef - Esa, F.; Tasirin, S.M. and Rahman, N.A. Agriculture and Agricultural Science Procedia 2014, 2, 113-119.
CrossRef - Ahmed, E.M. Journal of Advanced Research 2015, 6(2), 105-121.
CrossRef - Ibrahim, S.M.; Salmawi, K.M.E. and Zahran, A.H. Journal of Applied Polymer Science 2007, 104(3), 2003-2008.
CrossRef - Sannino, A.; Pappadà, S.; Madaghiele, M.; Maffezzoli, A.; Ambrosio, L. and Nicolais, L. Polymer 2005, 46(25), 11206-11212.
CrossRef - Caló, E. and Khutoryanskiy, V.V. European Polymer Journal 2015, 65, 252-267.
CrossRef - Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J. and Kenny, J.M. Polymer Degradation and Stability 2014, 107, 139-149.
CrossRef - Sonia, A. and Priya Dasan, K. Journal of Food Engineering 2013, 118(1), 78-89.
CrossRef - Murthy, P.S.K.; Murali Mohan, Y.; Varaprasad, K.; Sreedhar, B. and Mohana Raju, K. Journal of Colloid and Interface Science 2008, 318(2), 217-224.
CrossRef - Zhang, J.; Wang, L. and Wang, A. Macromolecular Materials and Engineering 2006, 291(6), 612-620.
CrossRef - Akhtar, M.F.; Hanif, M. and Ranjha, N.M. Saudi Pharmaceutical Journal 2016, 24(5), 554-559.
CrossRef - Navarra, M.; Dal Bosco, C.; Serra Moreno, J.; Vitucci, F.; Paolone, A. and Panero, S. Membranes 2015, 5(4), 810.
CrossRef - Sannino, A.; Demitri, C. and Madaghiele, M. Materials 2009, 2(2), 353.
CrossRef - Yoshimura, T.; Matsuo, K. and Fujioka, R. Journal of Applied Polymer Science 2006, 99(6), 3251-3256.
CrossRef - Esposito, F.; Nobile, M.A.D.; Mensitieri, G. and Nicolais, L. Journal of Applied Polymer Science 1996, 60(13), 2403-2407.
CrossRef - Stojanović, Ž.; Jeremić, K.; Jovanović, S. and Lechner, M.D. Starch – Stärke 2005, 57(2), 79-83.
CrossRef - Ul-Islam, M.; Khan, T. and Park, J.K. Carbohydrate Polymers 2012, 88(2), 596-603.
CrossRef - Maria, L.C.S.; Santos, A.L.C.; Oliveira, P.C.; Valle, A.S.S.; Barud, H.S.; Messaddeq, Y. and Ribeiro, S.J.L. Polímeros 2010, 20, 72-77.
CrossRef - Dahman, Y.; Jayasuriya, K.E. and Kalis, M. Applied Biochemistry and Biotechnology 2010, 162(6), 1647-1659.
CrossRef - Yue, L.; Zheng, Y.; Xie, Y.; Liu, S.; Guo, S.; Yang, B. and Tang, T. RSC Advances 2016, 6(73), 68599-68605.
CrossRef - Anbergen, U. and Oppermann, W. Polymer 1990, 31(10), 1854-1858.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









