Microwave Synthesis, Characterization and Antimicrobial Activity of Some Chromene Derivatives
R. Nandhikumar1,2  and K. Subramani1,3
and K. Subramani1,3
1R and D Centre, Bharathiar University Coimbatore, Tamilnadu, India.
2Department of Chemistry, The Kavery Engineering College, Salem, Tamilnadu, India.
3PG and Research Department of Chemistry Islamiah College, Vaniyambadi, Tamilnadu, India.
Corresponding Author E-mail: nandhikumar@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/3404051
Article Received on : 14-04-2018
Article Accepted on : 25-06-2018
Article Published : 12 Jul 2018
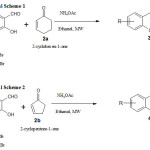
In the present study the synthesis of Chromene derivatives 7-ethyl-2, 3, 4, 4a-tetrahydro-1H-xanthen-1-one (3a), 7-bromo-2, 3, and 4, 4a-tetrahydro-1 H-xanthen-1-one (3b), 5-ethoxy-2, 3, and 4, 4a-tetrahydro-1 H-xanthen-1-one (3c),7-ethyl-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4a), 7-bromo-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4b),and 5-ethoxy-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4c) have been reported by the reaction of substituted Salicylaldehyde with 2-cyclopenten-1-one/2-cyclohexen-1-one in the presence of ammonium acetate with ethanol using microwave-irradiation. The Microwave synthesized complexes have been categorized via IR, 1H-NMR, 13C-NMR, Mass Spectroscopy then elemental analysis. In this Microwave irradiation method contains two scheme, in the Scheme 2 gives high yield compare to Scheme 1.In the antibacterial and anti-fungal study the compounds 3b, 4a shows good and 4b shows very good activity against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus and 3b, 3c shows good activity against Aspergillus Niger, Penicillium species and Candida albicans, compare to others. Short reaction time, environmentally friendly products, high economical, excellent yield and good microbial activity are the highest benefits of this technique which create it high commercial than the other methods.
KEYWORDS:Antibacterial; Antifungal; Cyclopenten-1-one; 2-Cyclohexen-1-one; Microwave
Download this article as:| Copy the following to cite this article: Nandhikumar R, Subramani K. Microwave Synthesis, Characterization and Antimicrobial Activity of Some Chromene Derivatives. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Nandhikumar R, Subramani K. Microwave Synthesis, Characterization and Antimicrobial Activity of Some Chromene Derivatives. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47274 |
Introduction
Eco-friendly synthetic procedure is a novel and promptly developing methods of medicinal chemistry. It’s very fast growing importance is used for medicinal chemistry research fields because these methods are constructed avoid the chemical waste and save the time. Microwave irradiation1-3 method is the greatest changes for traditional chemical synthetic root. Through applying the eco-friendly synthetic technique, we can eliminate the use of poisonous thinners, hazardous, producing substance and also the elimination of by-product. Gedye with Giguere (1986) stated for the paramount period that a natural organic synthesis might be directed precisely quickly under microwave synthesis methods. In the medicinal chemistry and natural products field 2H-Chromenes (2H-1-Benzopyrans) are acting as an important intermediate4-5 for many organic synthesis. Generally some Chromene containing compound used for the intermediates of positive logically arising product, such as Miroestrol compound. Coumarins compounds act as a very good antioxidant6 and equally fluorescent methods, pointers for custom in evaluating, stabilizers, besides with medicinal usage for their, diuretic, anticoagulant, anti HIV7, antitumor, anti-inflammatory,8-10 anticanser11, anti-Alzheimer’s,12-13 central nervous system activity14 anti-leukemic, anti-bacterial,15-25 anti-malarial events,26 emetic.27 Furthermore, they can besides be working as greasepaints and colors and used as possible eco-friendly agrochemicals. It has been reported28-32 that poly functional vinylic compounds can be prepared by using Ammonium acetate substrates. The structures of microwave synthesized Chromene derivatives assumed from their elemental analysis (C, H, O, N, and Br) and IR, 1H, 13C -NMR, Mass spectra and tested the antimicrobial activity. In our knowledge this tecnique is the best methods for microwave synthesis and microbial activity of Chromene derivatives because this is eco-friendly, simple method, good yield, enough short time and more economic.
Experimental
Use the open capillary tube to find out the compound melting points. FT-IR spectra data (KBR) were taken on Perkin-Elmer 1300 FT IR spectrometer and Bruker WM-400 instruments (FT NMR 400 MHz) recorded 1H NMR & 13C NMR (Internal standard- TMS), GC-MS spectra were recorded on GC-Mass spectrometer instruments. Total products presented suitable small analytical report. Cleanliness of the product was tested by thin layer chromatography methods.
Synthesis of 7-ethyl-2, 3, 4, 4a-tetrahydro-1H-xanthen-1-one (3a)
With a mixture of 5 mm 5-ethyl-2-hydroxybenzaldehyde in ethanol (few ml) and 20 mole% of Ammonium acetate followed by of 2-cyclohexen-1-one (10 mm) added and the constant stirring continued on Microwave irradiation for 5 minute. Then the reaction compound was converted into an acid medium with a few ml of concentrated HCl and the bottom level was detached, wash the aqueous level with DCM. The compound deposit was dehydrated and, after the distillation process, providing the Chromene compound 7-ethyl-2, 3, 4, 4a-tetrahydro-1H-xanthen-1-one (3a) and in good yield (Table 1, Scheme 1).
7-ethyl-2, 3, 4, 4a-tetrahydro-1H-xanthen-1-one (3a): (Scheme-1)
M.P of the product is 172ºC yield 58 %. IR (KBr,ʋmax, cm-1): 3116, 2995 (Ar-CH, str), 2860 (C-H str for CH3), 2846-2816 (C-H str for CH2), 1647 (C=O, str), 1534, 1575 (C=C, str), 1421, 1460 (C-C single bond str), 1220, 1290 (C-O single bond str). 1HNMR (DMSO-H2O) : 8.15 (1H , s), 7.55 (1H, s), 7.03-7.04 (1H , d), 6.66-6.67 (1H , d), 5.03-5.04 (1H, t), 3.28-3.34 (2H ,t),2.22-2.36 (2H, q),1.67-1.74 (2H, m),1.65-1.68 (2H , m),1.51-1.53(3H,t).13NMR (DMSO-H2O): 17.73, 23.84, 30.52, 35.13, 42.74, 59.14, 99.29, 107.01, 126.22, 127.21,128.33,131.93, 144.83, 148.28, 165.31. GC-Mass: m/z = 228 M+.
Synthesis of 7-Bromo-2, 3, and 4, 4a-tetrahydro-1 H-xanthen-1-one (3b)
With a mixture of 5 mm 5-Bromo-2-hydroxybenzaldehyde in ethanol (few ml) and 20 mole% of Ammonium acetate followed by of 2-cyclohexen-1-one (10 mm) added and the constant stirring continued on Microwave irradiation for 5 Min. Then the reaction compound was converted into an acid medium with a few ml of concentrated HCl and the bottom level was detached, wash the aqueous level with DCM. The compound deposit was dehydrated and, after the distillation process, providing the Chromene compound 7-bromo-2, 3, and 4, 4a-tetrahydro-1 H-xanthen-1-one (3b)) and in good yield (Table 1, Scheme 1).
7-Bromo-2, 3, and 4, 4a-tetrahydro-1 H-xanthen-1-one (3b): (Scheme-1)
M.P of the product is 213ºC yield 56 %. IR (KBr, ʋmax, cm-1): 3135, 3014 (Ar-CH str), 2859, 2843 (C-H str for CH2), 1702 (C=O group Str), 1560,1527 (C=C group str), 1384,1365 (single bond C-C str), 1221,1279 (C-O str.),1869 (overtone for halide substitution) 1HNMR (DMSO-H2O) : 8.299 (1H ,s), 7.89 (1H, s),7.348-7.378 (1H ,d) 5.272-5.283 (1H, t), 3.972-3.983 (2H , t), 2.37-2.39 (2H, multiplet), 1.66-1.78(2H, m). 13NMR (DMSO-H2O) 17.76,23.61,32.88,59.21,98.85,112.68,121.07,128.15,129.94,131.17,143.76, 148.65, 165.17. GC-Mass: m/z = 227. M+.
Synthesis of 5-ethoxy-2, 3, 4, 4a-tetrahydroxanthen-1-one (3c)
With a mixture of 5 mm 3-ethoxy-2-hydroxybenzaldehyde in ethanol (few ml) and 20 mole% of Ammonium acetate followed by of 2-cyclohexen-1-one (10 mm) added and the constant stirring continued on Microwave irradiation for 5 Min. Then the reaction compound was converted into an acid medium with a few ml of concentrated HCl and the bottom level was detached, wash the aqueous level with DCM. The compound deposit was dehydrated and, after the distillation process, providing the Chromene compound 7-ethyl-2, 3, 4, 4a-tetrahydro-1H-xanthen-1-one (3a) and in good yield (Table 1, Scheme 1).
5-ethoxy-2, 3, 4, 4a-tetrahydroxanthen-1-one (3c): (Scheme-1)
M.P of the product is 197ºC yield 62 %. IR (KBr, ʋmax, cm-1): 3186, 3070 (Ar-CH str.), 2955, 2830 (C-H str, CH2), 1688 (aromatic C=O Str), 1526, 1474 (C=C group str), 1347, 1316 (C-C group str), 1266, 1223 (C-O str). 1HNMR(DMSO-H2O): 7.98 (1H,s), 7.396-7.399 (1H , d), 7.376-7.393(1H, q), 7.265-7.271 (1H, d), 5.181-5.253(1H, t), 4.512-4.588 (2H, q), 3.172-3.381 (2H),2.476-2.599 (2H ,m),2.453-2.589 (2H ,m),2.211-2.232 (3H,t). 13HNMR (DMSO-H2O):17.68, 23.29, 29.05, 33.65, 53.28, 59.05, 112.29, 116.26, 118.46, 121.16, 134.25, 147.50, 149.65, 152.25, 165.46. Mass spectra: m/z = 244 M+ (base peak).
Synthesis of 7-ethyl-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4a)
With a mixture of 5 mm 5-ethyl-2-hydroxy benzaldehyde in ethanol (few ml) and 20 mole% of Ammonium acetate followed by of 2-cyclopenten-1-one (10 mm) added and the constant stirring continued on Microwave irradiation for 5 Min. Then the compound was converted into an acid medium with a few ml of concentrated HCl and the bottom level was detached, wash the aqueous level with DCM. The organic compound deposit was dehydrated and, after the distillation process, providing the Chromene compound 7-ethyl-3, 3a-dihydrocyclopenta[b]Chromen-1(2H one( 4a) andin good yield.(Table 2, Scheme 2).
 |
Scheme 1,2 |
7-ethyl-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4a): (Scheme-II)
M.P of the product is 167ºC yield 66 %. IR (KBr, ʋmax, cm-1): 3119, 3010 (Ar-CH str.), 2976 (C-H str for CH3), 2904, 2816 (C-H str for CH2) 1667 (aromatic C=O group str), 1544, 1450 (C=C group str), 1365 (C-C group str), 1223, 1173(C-O group str). 1HNMR (DMSO-H2O): 7.751 (1H , s), 7.334-7.347 (1H, d), 7.269-7.279 (1H, d), 6.951 (1H, s), 5.133-5.247 (1H, t), 4.28-4.34 (2H, t), 3.118-3.166 (2H ),2.671-2.729 (2H,m),2.229-2.366 (3H, m).13C NMR (DMSO-H2O):17.74, 23.74, 31.92, 34.83, 59.94, 103.11, 11.12, 112.01, 126.11, 128.33,141.84, 144.89, 148.15, 168.32.GC-Mass: m/z = 214 M+.
Synthesis of 7-ethyl-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4b)
With a mixture of 5 mm 5-bromo-2-hydroxybenzaldehyde in ethanol (few ml) and 20 mole% of Ammonium acetate followed by of 2-cyclopenten-1-one (10 mm) added and the constant stirring continued on Microwave irradiation for 5 Min. Then the compound was converted into an acid medium with a few ml of concentrated HCl and the bottom level was detached, wash the aqueous level with DCM. The organic compound deposit was dehydrated and, after the distillation process, providing the Chromene compound 7-bromo-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4b) and in good yield. (Table 2, Scheme 2).
7-bromo-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4b): (Scheme-II)
M.P of the product is 204 ºC yield 64 %. IR (KBr, ʋmax, cm-1): 3157, 3021 (Ar-CH str), 2964, 2832 (C-H str, CH2), 171O (aromatic C=O group str), 1573, 1526 (C=C group str), 1465, 1391 (C-C group str), 1197, 1250 (C-O str.), 1895(overtone for halides). 1HNMR (DMSO-H2O): 8.061 (1H, s), 7.683 (1H, s), 7.511-7.578 (1H, d), 7.499-7.508 (1H, d), 5.371-5.379 (1H, t), 3.029-3.166 (2H, t), 2.265-2.389 (2H, t). 13NMR (DMSO-H2O): 33.06,36.00,73.12,106.88,112.55,115.18,128.33,128.65,123.88,133.06,148.89,172.12.GC-Mass: m/z = 264 M+.
Synthesis of 7-ethyl-3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4c)
With a mixture of 5 mm 3-ethoxy-2-hydroxybenzaldehyde in ethanol (few ml) and 20 mole% of Ammonium acetate followed by of 2-cyclopenten-1-one (10 mm) added and the constant stirring continued on Microwave irradiation for 5 Min. Then the compound was converted into an acid medium with a few ml of concentrated HCl and the bottom level was detached, wash the aqueous level with DCM. The organic compound deposit was dehydrated and, after the distillation process, providing the Chromene compound 5-ethoxy -3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4c) and in good yield. (Table 2, Scheme 2).
5-ethoxy -3, 3a-dihydrocyclopenta[b]Chromen-1(2H)-one (4c): (Scheme-II)
M.P of the product is 187ºC yield 63 %. IR (KBr, ʋmax, cm-1): 3179, 3094, 3027 (Ar-CH str.), 2988, 2931(C-H str for CH3), 2887(C-H str for CH2)1667 (aromatic C=O group str), 1594, 1532 (C=C group str), 1475, 1376 (C-C group str), 1189, 1275 (C=O group str). 1HNMR (DMSO-H2O): 7.555 (1H, s), 7.213 (1H, d), 6.813-6.935 (1H, q), 6.660-6.683 (1H, d), 4.86-4.91 (1H, t), 3.95-4.06 (2H, q), 3.53-3.61 (2H, t), 2.48-2.72(2H, m), 2.22, 2.24(3H, t). 13NMR (DMSO-H2O): 17.88, 30.31, 34.11, 59.00, 68.25, 111.67, 112.19, 115.55, 116.05,129.46, 138.26,141.76,144.73,169.96. GC-Mass: m/z = 230 M+.
Results and Discussion
The green synthesis of all sequences of the final product was prepared by the reaction Substituted Salicylaldehyde and Ammonium acetate followed by of 2-cyclohexen-1-one added in the presence of Microwave irradiation. Formation of 3a-3c was established by the occurrence of C-O-C extending peaks at 1279,1266 &1290, ʋmax, cm-1 and aromatic C=O group stretching peaks at 1647,1702&1688, ʋmax, cm-1 in IR and aromatic group singlet at 7.75,8.29&7.98, ʋmax, cm-1 for the xanthene cyclic group in 1HNMR spectra.
1H-NMR spectrum valued showed a fine triplet at δ 5.03,5. &5.18 due to a xanthene group of their structure were found over spectral values and physical data (Table-1). FT-IR and 1H NMR spectral records exposed carbonyl group absorption band at 1702,1688 &1647, ʋmax, cm-1 of the aromatic -CO-C group, aromatic C-O extends band at 1290,1279 &1266, ʋmax, cm-1 aliphatic group C-H and aromatic C-H group stretching at 2860,2859 &2830 and 3116,31357 & 3186, ʋmax, cm-1 group of xanthene molecule. GC-Mass values also reinforced the planned structure through showing base peak at m/z = 228,277 & 244M+.
Table 1: Chromene compounds physical and analytical data (3a, 3b&3c).
|
Compounds |
Mol. F |
Mol.W |
Y (%) |
M.P (0C) |
Time (Min) |
Found/calculated (%) |
|||
|
C |
H |
O |
Br |
||||||
|
3a |
C15H16O2 |
228 |
58 |
172 |
5 |
78.92 (78.84 |
7.06 6.98 |
14.02 13.86 |
0.00 0.00) |
|
3b |
C13H11BrO2 |
227 |
56 |
213 |
5.5 |
55.94 (55.82 |
3.97 3.81 |
11.46 11.48 |
28.63 28.71) |
|
3c |
C15H16O3 |
244 |
62 |
197 |
6 |
73.75 (73.66 |
6.60 6.52 |
19.65 19.52 |
0.00 0.00) |
In other methods, the reaction of Substituted Salicylaldehyde and Ammonium acetate followed by of 2-cyclopenten-1-one added in the presence of Microwave irradiation. Formation of 4a, 4b and 4c was established by the occurrence of C-O-C group stretching peaks at 1223,1275&1250, ʋmax, cm-1 and C=O is stretching peaks at 1667,1667.41&1710, ʋmax, cm-1 in FT-IR and aromatic group singlet at 7.751,8.06&7.55 cm-1 for the Chromene cyclic group in 1HNMR spectra.
Table 2: Chromene compounds physical and analytical data (4a, 4b&4c).
|
Compounds |
Mol. F |
Mol.W |
Y (%) |
M.P (0C) |
Time (Min) |
Found/Calculated (%) |
|||
|
C |
H |
O |
Br |
||||||
|
4a |
C14H14O2 |
214 |
66 |
167 |
4 |
78.48 (78.38 |
6.59 6.48 |
14.93 14.83 |
0.00 0.00) |
|
4b |
C12H9BrO2 |
263 |
64 |
204 |
4.5 |
54.37 (54.25 |
3.42 3.32 |
12.07 11.98 |
30.14 30.02) |
|
4c |
C14H14O3 |
230 |
63 |
187 |
5 |
73.07 (72.96 |
6.13 6.08 |
20.85 20.75 |
0.00 0.00) |
1H-NMR range presented a fine triplet peaks at δ 5.13,5.37&4.86 due to Chromene compounds of their structure were found over spectral and physical data (Table-2). FT-IR and 1H-NMR spectral data exposed carbonyl group absorption band at 1667,1710&1667 cm-1 of the aromatic-CO-C group, aromatic C-O is stretching band at 1223, 12791250 & 1250 cm-1 aliphatic group C-H and aromatic group C-H stretching at 2816,2832&2887 and 3119,3157&31863179 cm-1 group of Chromene molecule. GC-Mass data also reinforced the planned structure by showing base peak at m/z = 214, 264&230M+.
Antibacterial Activity
Novel synthesized Chromene derivatives are selected for their anti-bacterial behavior in vitro beside the species of Escherichia coli (Gram –ve, micro-organism), Pseudomonas aeruginosa (Gram –ve, micro-organism) and Staphylococcus aureus (Gram +ve, micro-organism) by agar well (disk) diffusion techniques. Ciprofloxacin is used as a standard drug and the outcomes are shown in Table-3 and Fig.1. In the antibacterial study the compounds 3b, 4a shows good and 4b shows very good activity against Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa, compare to others.
Table 3: Antibacterial activity for Chromene compounds (3a-3c & 4a-ac).
|
Antibacterial activity for (3a-3c &4a-4c)mm |
|||
|
Compounds |
Escherichia coli (mm) |
Staphylococcus aureus(mm) |
Pseudomonas aeruginosa(mm) |
|
3a |
6 |
6 |
5 |
|
3b |
7 |
6 |
4 |
|
3c |
8 |
7 |
4 |
|
4a |
7 |
4 |
5 |
|
4b |
16 |
14 |
12 |
|
4c |
5 |
4 |
4 |
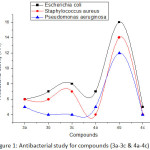 |
Figure 1: Antibacterial study for compounds (3a-3c &4a-4c).
|
Anti-fungal Activity
Novel green synthesized Chromene compounds are selected for their antifungal behavior in vitro beside the micro-organism of Penicillium species, Aspergillus Niger and Candida albicans, by agar well (disk) diffusion techniques. The experiment products are liquefied in DMSO.
Table 4: Anti-fungal activity for (3a-3c &4a-4c).
|
Anti-fungal activity for (3a-3c &4a-4c)mm |
|||
|
Compounds |
Penicillium sps (mm) |
Aspergillus niger (mm) |
Candida albicans (mm) |
|
3a |
– |
– |
– |
|
3b |
8 |
4 |
– |
|
3c |
4 |
5 |
5 |
|
4a |
5 |
– |
– |
|
4b |
7 |
– |
– |
|
4c |
7 |
– |
– |
 |
Figure 2: Anti-fungal study for compounds (3a-3c &4a-4c).
|
Commercial Amphotericin-B is used as a standard and the outcomes are displayed in Table-4 and Fig.2. In the anti-fungal study the compounds 3b and 3c shows very good activity against Aspergillus Niger, Penicillium species, Candida albicans and, compare to others.
Conclusions
Microwave assisted synthesis of Substituted Salicylaldehyde with 2-cyclopenten-1- /2-cyclohexen-1-one in the presence of ammonium acetate with ethanol gives a good yield of some novel Chromene compounds (3a-3c and 4a-4c). In this synthetic Microwave irradiation method the compound 4a-4c (Table 1) gives high yield. Total compounds are lively against all the verified bacterial and fungal strains. Compound 3a-3c and 4a-4c are medium or more active against antibacterial and fungal strains for standard drug for antibacterial activity. In the antibacterial and anti-fungal study the compounds 3b, 4a shows good and 4b shows very good activity against Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli and 3b, 3c shows very good activity against Aspergillus Niger, Penicillium species and Candida albicans, compare to others. This research work has high economic, because used available and simple catalyst, simple reaction methods, need less time and get ecofriendly nature.
Acknowledgements
The authors are thankful to the director, Bharathiyar university R&D center for giving the opportunity to complete the research and for their constant encouragement to write the article in good manner and Dr. G. Sarawathi, HOD of chemistry Government Engineering College Bargur, Tamilnadu, India for their encouragement and providing necessary research facilities.
Reference
- Vrushali Patil.; Ashish Asrondkar.; Anil Bobade.; Abhay Chowdhary.; IJSR. 2013, 1688-1690.
- Shivaputra, A.; Patil,Siddappa A Patil & Renukadevi Patil, Future Med Chem., 2015, 7.
- Abolghasem shameli akandi.; ebrahim balali.; talieh mosavat.; mohammad mehdi ghanbari.; and ali eazabadi. Orient. J. Chem., 2014, 30(2), 587-591.
- S. Arulmurugan, S.; Helen P. Kavitha and Venkatraman,B.R. Rasayan J.Chem., 2010, 3(3) 385-410.
- Gudipudi Gopinath.; Venu Sankeshi.; Shaymperugu.; Malini D. Alaparthi.; Srinivas Bandaru.; Vijay K. Pasala.; Prasad Rao Chittineni.; David Krupadanam, G.L.; Someswar R. Sagurthi. European J of Med Chem., 2016, 124(29), 750-762.
CrossRef - Jessica Elizabeth.; Philip Shanty.; Angamaly Antony Sneha.; Jose Eeettinilkunnathi.; M.R. Prathapachandra Kurup, M.R.; Mohanan Puzhavoorparambi.; Velayudhan. Inorganica Chimica Acta., 2018, 469(1), 87-97.
- Nancy Thomas and Subin Mary Zachariah. Asian J Pharm Clin Res., 2013, 6(2), 11-15.
- Shu-Ting Chung.; Wen-Hsin Huang.; Chih-Kuo Huang.; Feng-Cheng Liu.; Ren-Yeong Huang.; Chin-Chen Wu.; An-Rong Lee.; Research on Chemical Intermediates., 2016, 42(2), 1195–1215.
- Vrushali Patil.; Ashish Asrondkar.; Anil Bobade.; Abhay Chowdhary.; IJSR., 2013, 2319-7064.
- Bahubali M.Chougala.; S.Samundeeswari.; Megharaja Holiyachi.; Nirmala S.Naik.; Lokesh A.Shastri.; Suneel Dodamani.; SunilJalalpure .; Sheshagiri R. Dixit.; Shrinivas D. Joshi .; Vinay A. European J of Med Chem., 2018, 143(1),1744-1756.
- Jawaid Akhtar Ahsan.; Ahmed Khan Zulphikar.; Ali Rafi Haider.; Shahar Yar, M. European J of Med Chem., 2017, 125(5), 143-189.
- Islam H. El Azab.; Mohamed M. Youssef and Mahmoud A. Amin . Molecules., 2014, 19, 19648-19664.
- Sujay Laskar and Goutam Brahmachari. Signpost Open Access J. Organic & Biomol Chem., 2014, 2, 1-50.
- Rupnar, B.D.; Rokade P. B.; Gaikwad, P. D and Pangrikar, P.P. IJCEBS., 2014, 2(1), 2320–4087.
- Jessica Elizabeth.; Philip Shanty.; Angamaly Antony Sneha .; Jose Eeettinilkunnathi.; Prathapachandra Kurup Mohanan, M.R.; Puzhavoorparambi.; Velayudhan.Inorganica Chimica Acta., 2018, 469(1), 87-97.
- Latha Rani, N.; Manasagangotri.; Mysuru.; Prashanth,T.; Sridhar, M.A.; Gurupadaswamy,H.D.; Shaukath Ara, K& N. K. Lokanath N.K.2016, 11,262-270.
- Shweta Saxena.; Ratnesh das and Arti saxena. Chem Sci Rev Let., 2015, 4(14), 688-694.
- Lamia Dammak.; Mariem Kammoun.; Noureddine Allouche.; Houcine Ammar and Souhir Abid. Org. Commun., 2017, 10:1 32-39.
- Nirav K Shah.; Nimesh M Shah., Manish P Patel.; Ranjan G Patel, J of Chem Sci., 2013, 125(3), 525–530.
- Aziz Behrami, Orient. J. Chem., 2014, 30(4), 1747-1752.
CrossRef - Chetan B. Sangani.; Nimesh M. Shah.; Manish P. Patel and Ranjan G. Patel, J. Serb. Chem. Soc., 2012, 77 (9) 1165–1174.
- Sharaish, P.; Sivasubramanian, N.; Bollywar Archana Devi.; Madhulatha, C.; Sushma, G.S and Maithri, P. IJPLS., 2013, 4(3), 2458-2464.
- Hoda Pasdar.; Naser Foroughifar.; Bahare Hedayati Saghavaz.; J Med Microbiol Infec Dis., 2015, 3 (3-4), 75-79.
- Pankaj S. Chaudhari.; Shrikant S. Patil. Int J of Chem Tech Res, 2017, 10(15), 126-134.
- Venkataraman, S.; Meera,R.; Vidya Ramachandran.; Nagarajan,K.; Aruna,A.; Padma Thanga Parameswari,S and Devi,P.; IJRPC., 2013, 3(4).
- A.Parthiban, A.; Muthukumaran,J. AshanManhas.; Kumkum Srivastava.; Krishna,R.; Surya Prakash Rao,H.; Bio & Med Chem Lett., 2015, 25(20), 4657-4663.
CrossRef - Islam H. El Azab.; Mohamed M. Youssef and Mahmoud A. Amin. Molecules., 2014, 19, 19648-19664.
- Nirav.K.Shah.; Nimesh.M Shah.; Manish P Patel, Ranjan G Patel, Chem .sci.,2013, 125, 525-530.
- Jian-Feng Zhou.; Yuan-Zhi Song.; Jin-Shun Lv.; Gui-Xia Gong.; Shujiang Tu .Int. J. of Rapid comm.syn org chem., 2009, 8,112.
- Jian-Feng Zhou.; Xiao-Jun Sun.; Feng-Wen Lou Meng Lv.; Lu-Lu Zhang.; Res Chem Intermed., 2013, 39, 1401–1408.
CrossRef - Kanakaraju, S.; Prasana Srinivas Basavoju, B.; Chandramouli, G.V.P. Arb J chem., 2017, 10(2), 2705-2713.
- Ahmed Khodairy.; Ali, M.; Ali, T.; Mohamed, El-Wassimy. J of Het Chem., 2017, 5 4,3342–3349.

This work is licensed under a Creative Commons Attribution 4.0 International License.









