Impervious Nature of Al2O3-PANI Composite Against Corrosion on Mild Steel in Strong Acidic Environment
P. Kamatchi Selvaraj , S. Sivakumar and S. Selvaraj
, S. Sivakumar and S. Selvaraj
P.G and Research Dept. of Chemistry, Govt. Arts College for Men (AUT), Nandanam, Chennai – 35, India.
Corresponding Author E-mail: porbal96@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/3404017
Article Received on : 19-06-2018
Article Accepted on : 11-07-2018
Article Published : 14 Aug 2018
Solubility problem of composite in aqueous medium is resolved on adding Al2O3 during the oxidative polymerization of aniline using ammonium peroxydisulphate as an oxidant and sodium salt of dodecyl bezenesulphonic acid as surfactant and dopant at 273 K temperature. The yielded water soluble Al2O3-PANI composite is confirmed by comparing the FTIR, XRD and SEM recorded spectra with previously reported one. Gravimetric method exposes that the prepared composite is having confrontation against corrosion. Only a slight change in efficiency on continuous exposure up to eight hours is observed. OCP data are transformed in to potential graph to exposes the invincibility of the composite against corrosion. Measurement of potentiodynamic polarization and EIS studies also confirms the defiance against corrosion.
KEYWORDS:Al2O3-PANI; EIS; Mild Steel; OCP; Potentiodynamic Polarization
Download this article as:| Copy the following to cite this article: Selvaraj P. K, Sivakumar S, Selvaraj S. Impervious Nature of Al2O3-PANI Composite Against Corrosion on Mild Steel in Strong Acidic Environment. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Selvaraj P. K, Sivakumar S, Selvaraj S. Impervious Nature of Al2O3-PANI Composite Against Corrosion on Mild Steel in Strong Acidic Environment. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48375 |
Introduction
Metalworking industries suffers mishap due to chemical or electrochemical attack on metal. Metal corrosion is a resolute threat to national economy and industry design.1,2 It is miserable that corrosion can’t be completely eradicated, as it is a natural process. Corrosion activities may be slow downed by other dynamic and/or substitute mechanisms. The undeniable methods include cathodic protection,3,4 protective coating5 and addition of inhibitors.6 Most of the protective coating layers lost their identity on prolonged exposure to corrosive environment .7-9 Corrosion resistive performance of epoxy coatings were improved on insertion of TiO2 nanomaterials.10,11 It is a well known fact that corrosion of aluminum utensils is prevented due to the formation of protective Al2O3 layer on the surface of aluminum. Utility of Al2O3 have been found in the activities of catalyst,12 retardant for fire,13 absorbent14 and filler.15 Stability against chemical action, good conducting property, idiosyncratic doping behavior of polyaniline and other applications of it in energy storage and sensors attracted significantly to employ it as inhibiting material.16-18 The structural morphology, thermal property and conductivity of PANI varied on insertion of TiO2 and Al2O3.19,20 Reinforced dielectric properties have been noticed for Al2O3-PANI compositie.20,21 Usage of solid Al2O3-PANI showed high value for dielectric property and impedance with very high Z“ values.22 All above observations stimulated to synthesis water soluble composite (Al2O3-PANI) and to evolve its capacity to diminish corrosion of carbon steel in strong acidic environment.
Materials and Instrumentation
Materials
AR grade monomer (aniline) was procured from Merck Ltd. After adding a pinch of zinc dust, it was purified by distillation to employ for polymerization. Phosphoric acid, Al2O3, ammonium peroxydisulphate (APS) and sodium salt (DBSA) chemicals with AR grade purchased from Merck Ltd. were used without purification.
Instrumentation
The infrared spectrum, in the frequency range of 4000-450 cm-1, was recorded by Perkin-Elmer 337 spectrometer. The XRD and SEM spectra were registered in Rigaku Maniflex diffractometer (Japan) and JSM-6390 Scanning Electron Microscope respectively. Potentiodynamic polarization studies and impedance measurements were documented in ECLAB 10.37 model. To observe the OCP values, CHI electrochemical analyzer instrument 1200B model was adopted.
Experimental
Synthesis of Al2O3 -Polyaniline Composite
Al2O3-PANI composite was synthesized by modified in-situ chemical oxidative polymerization method.23-25 Aniline (18.6g) was added to 1M solution of Phosphoric acid (200ml) and stirred for half-an-hour. Solution prepared by dispersing required amount of Al2O3 in100 ml of 0.1M DBSA using 42 kHz oscillation frequency for 45 minutes in a sonicator was mixed with aniline solution. The mixture was kept under constant stirring for two hours at 273 K along with addition of 200 ml of 1M APS solution in drops. Complete polymerization was achieved by stirring the above mixture for another three hours. The product (dark green in color) obtained was filtered, washed with deionized water, acetone, and dried in hot air oven at 55°C for 24 hours.
Results and Discussion
Characterization of Al2O3-PANI Composite
FTIR Analysis
The peak appears at 613 cm-1 in the FTIR spectrum of Al2O3 [Fig.1(a)] was assigned to Al-O stretching vibration and the peak arises around 3460 cm-1 was ascribed to O-H vibration mode.26,27 The bands appear in the FTIR spectrum of PANI [Fig. 1(b)] around 1562 cm-1 and 1447 cm-1 are due to stretching vibration of quinoid and benzenoid rings. The bands emerge about 1230 cm-1 and 1033 cm-1 are ascribed to C-N stretching vacillation.28,29 The N-H stretching of secondary amine fetch up near 3400 cm-1.
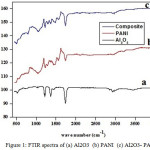 |
Figure 1: FTIR spectra of (a) Al2O3 (b) PANI (c) Al2O3- PANI. |
The FTIR spectrum of Al2O3-PANI composite [Fig. 1(c)] consist of the peaks due to Al2O3 and PANI with small blue or red shift. The O-H stretching of Al2O3 appears at 3490 cm-1. The C=C stretching mode of quinoid rings occur around 1550 cm-1 and 1490 cm-1. The Al-O stretching emerge at 508 cm-1. Above results implies that the PANI has been coated over the surface of Al2O3.22,30,31
XRD Analysis of PANI and Al2O3-PANI Composite
The XRD of Al2O3-PANI composite (Fig. 2b) contains various peaks assigned to alumina.32,33 The broad peak of PANI34 (Fig. 2a) centered between 2θ= 20°C-30°C is reappeared in the XRD of composite. This reveals the interaction of PANI with Al2O3.31
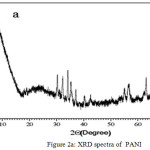 |
Figure 2a: XRD spectra of PANI. |
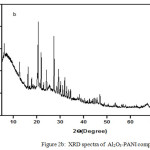 |
Figure 2b: XRD spectra of Al2O3-PANI composite. |
SEM Analysis of Al2O3 and Al2O3– PANI Composite
The SEM of metal oxide (Fig. 3a) gazes like a cluster with diameter ranging from 150 nm to 270 nm. The composite SEM (Fig. 3b) display flake structure having diameter ranging from 427 nm to 452 nm. This compeers to the previous work.30,31
 |
Figure 3: SEM spectra of (a) Al2O3 and (b) Al2O3-PANI composite. |
Preparation of Electrode Materials
Specimens fraternized with C: 0.21%, Si: 0.035%, Mn: 0.25%, P: 0.082% and 99.28% of Iron was trimmed into pieces measuring 4 cm x 2 cm x 0.2 cm and were scoured with disparate abrasive sheets starting from 600 grit to 1200 grit. The polished specimens were effaced by double distilled water, acetone and dried in a desiccators. Above freshly polished coupons were utilized in weight loss measurements.
Preparation of Electrolytic Solutions
The belligerent solutions of one molar and two molar sulphuric acid were prepared by diluting analytical quality sulphuric acid. Required amount (125-500ppm) of composite was added to the belligerent solution to obtain test solutions.
Evaluation of Inhibition Property
Assessment of Corrosion by Weight loss Measurement
Weight loss determination is the preliminary technique to measure corrosion. Freshly polished mild steel coupons were fully immersed in 250 ml of belligerent solutions and test solutions for eight hours continuously under room condition. The coupons were taken out at two hours time intervals, washed with bristle brush under tap water and then cleaned by distilled water, ethyl alcohol and acetone. After drying at room temperature, they were reweighed to assess the inhibition efficiency (IE %) and surface coverage (θ)35. The assessed values of IE and (θ) are provided in Table 1 and 2.
Table 1: IE and θ values assessed from the weight loss measurement in 1M belligerent and test solutions.
|
Conc. of Composite (ppm) |
2-hours |
4-hours |
6-hours |
8-hours |
||||||||
|
Weight loss (g) |
I.E (%) |
(θ) |
Weight loss (g) |
I.E (%) |
(θ) |
Weight loss (g) |
I.E (%) |
(θ) |
Weight loss (g) |
I.E (%) |
(θ) |
|
|
Blank |
0.1351 |
— |
— |
0.2275 |
— |
— |
0.2982 |
— |
— |
0.3666 |
— |
— |
|
125 |
0.0299 |
78 |
0.778 |
0.0534 |
76 |
0.765 |
0.0806 |
72 |
0.724 |
0.1080 |
70 |
0.705 |
|
250 |
0.0272 |
80 |
0.798 |
0.0484 |
79 |
0.787 |
0.0615 |
79 |
0.793 |
0.0965 |
73 |
0.736 |
|
375 |
0.0180 |
87 |
0.866 |
0.0332 |
85 |
0.854 |
0.0551 |
81 |
0.815 |
0.0930 |
75 |
0.746 |
|
500 |
0.0158 |
88 |
0.883 |
0.0301 |
87 |
0.867 |
0.0510 |
82 |
0.825 |
0.0715 |
80 |
0.804 |
Table 2: IE and θ values assessed from the weight loss measurement in 2M belligerent and test solutions.
|
Conc. of Composite (ppm) |
2-hours |
4-hours |
6-hours |
8-hours |
||||||||
|
Weight loss (g) |
I.E (%) |
(θ) |
Weight loss (g) |
I.E (%) |
(θ) |
Weight loss (g) |
I.E (%) |
(θ) |
Weight loss (g) |
I.E (%) |
(θ) |
|
|
Blank |
0.2412 |
— |
— |
0.4172 |
— |
— |
0.5507 |
— |
— |
0.7046 |
— |
— |
|
125 |
0.0748 |
69 |
0.689 |
0.1388 |
67 |
0.667 |
0.1901 |
65 |
0.654 |
0.2609 |
63 |
0.629 |
|
250 |
0.0680 |
72 |
0.718 |
0.1261 |
70 |
0.697 |
0.1850 |
67 |
0.678 |
0.2404 |
65 |
0.652 |
|
375 |
0.0631 |
74 |
0.738 |
0.1142 |
72 |
0.724 |
0.1668 |
70 |
0.698 |
0.2226 |
68 |
0.684 |
|
500 |
0.0522 |
78 |
0.783 |
0.1010 |
76 |
0.757 |
0.1402 |
74 |
0.745 |
0.2016 |
71 |
0.714 |
Attentive examination of the data’s existing in the Tables 1 and 2 discloses the substantial conservative nature of Al2O3-PANI composite against corrosion. Minimal changes in efficacy observed even after eight hours committed that the prepared water soluble composite have good resistivity versus corrosion.
Open Circuit Potential
The OCP values up to 120 minutes were recorded using CHI Electrochemical analyzer 1200B model. A cell comprise of working electrode made from mild steel having 1 cm2 area, saturated calomel electrode as reference electrode and platinum electrode as counter electrode was employed to measures the OCP statistics. The perceived information are given in Fig. 4 and 5.
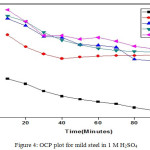 |
Figure 4: OCP plot for mild steel in 1 M H2SO4 |
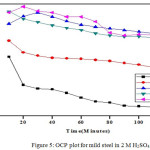 |
Figure 5: OCP plot for mild steel in 2 M H2SO4 |
Shift of OCP points to positive potential value on addition of Al2O3-PANI composite implies the resistive character.36,28
Electrochemical Measurements
Potentiodynamic polarization and EIS studies were detected in EC-LAB analyzer model 10.37 instrument assembled with three electrode compartment cell. Specimen, having 1cm2 area and the remaining area covered with araldite epoxy resin, cut from ASTM 415 mild steel was used as working electrode. Calomel electrode and Platinum electrode were used as reference electrode and counter electrode respectively. On maintaining the potential between -200 to +200 m V with scan rate of 0.5m V s-1, the potentiodynamic polarization studies were documented. Impedance measurements were executed with 10 mV AC sine wave amplitude in the frequency range of 100 kHz – 10 mHz.
Potentiodynamic Polarization Measurements
The parameters such as Icorr, Ecorr, bc and ba and surface coverage (θ) area measured from the Tafel plots given in Fig. 6 and 7 are bestowed in the Table 3 and 4 respectively. Reported formula37 was used to calculate the resistivity of Al2O3-PANI composite.
 |
Figure 6: Tafel plots of mild steel in 1M belligerent and test solutions. Click here to View figure |
Table 3: Corrosion resistive parameters for Mild Steel in 1M belligerent and test solution.
|
Conc. of Composite (ppm) |
-ECorr (mV vs. SCE) |
ba (mV dec-1) |
bc (mV dec-1) |
Icorr (µA cm-2) |
Inhibition Efficiency (%) |
Surface coverage (θ) |
|
Blank |
455 |
61 |
63 |
1960 |
— |
— |
|
100 |
484 |
36 |
40 |
1269 |
35 |
0.3525 |
|
200 |
483 |
33 |
37 |
997 |
49 |
0.4913 |
|
300 |
508 |
33 |
45 |
636 |
67 |
0.6755 |
|
400 |
496 |
21 |
23 |
616 |
69 |
0.6857 |
|
500 |
488 |
18 |
20 |
564 |
71 |
0.7122 |
 |
Figure 7: Tafel plots of mild steel in 2M belligerent and test solutions. |
Table 4: Corrosion resistive parameters for Mild Steel in 2M belligerent and test solution.
|
Conc. of Composite (ppm) |
-ECorr (mV vs. SCE) |
ba (mV dec-1) |
bc (mV dec-1) |
Icorr (µA cm-2) |
Inhibition efficiency (%) |
Surface coverage (θ) |
|
Blank |
439 |
44 |
57 |
2537 |
— |
— |
|
100 |
470 |
24 |
29 |
1733 |
32 |
0.3169 |
|
200 |
455 |
48 |
66 |
1510 |
40 |
0.4048 |
|
300 |
472 |
20 |
23 |
1399 |
45 |
0.4485 |
|
400 |
477 |
20 |
22 |
937 |
63 |
0.6306 |
|
500 |
480 |
20 |
21 |
904 |
64 |
0.6463 |
Increase in corrosion current is noticed with increase in the concentration of acid. The protection accomplishment of the composite is reflected in the steady fall in noticed Icorr value. Changes in efficiency on increasing the concentration of composite undeniably prove the resistivity of the composite. Inconsiderable changes observed in the Ecorr, ba and bc value presented in table 3 & 4 on varying the concentration of Al2O3-PANI disclose it as mixed type inhibitor.38
Electrochemical Impedance Measurements
Impedance parameters (Rct, Cdl) were calculated using the following equivalent circuit.
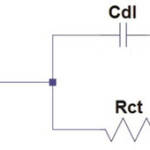 |
Scheme 1 |
Semicircle manifestation of Nyquist plots indicate the protection against corrosion and also reflects single charge transfer process.39 The decrease in flow of corrosion current reflected in the increase in diameter of capacitive loop. On increasing the concentration of composite, the diameter of the Nyquist plots increases in the Figures 8 and 9. This reveals that the composite gets adsorbed on the metal surface and prevents the flow of corrosion current and thereby acts as a better inhibitor in low pH environment. Previously reported formula37 is used to measure the inhibition efficiency.
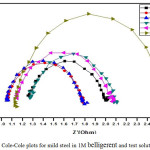 |
Figure 8: Cole-Cole plots for mild steel in 1M belligerent and test solution. |
Table 5: Impedance parameters for Mild Steel in 1M belligerent and test solution.
|
Conc. of Composite (ppm) |
Rs (Ω) |
Cdl (µ F cm-2) |
Rct (Ω cm2) |
Inhibition efficiency (%) |
Surface Coverage(θ) |
|
Blank |
1.076 |
643 |
0.5018 |
— |
— |
|
100 |
1.070 |
712 |
0.7583 |
34 |
0.3417 |
|
200 |
1.278 |
565 |
0.8841 |
38 |
0.4324 |
|
300 |
1.256 |
608 |
0.8961 |
44 |
0.4400 |
|
400 |
1.153 |
500 |
0.9932 |
50 |
0.5032 |
|
500 |
1.154 |
552 |
1.372 |
63 |
0.6350 |
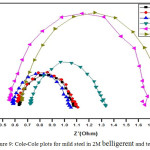 |
Figure 9: Cole-Cole plots for mild steel in 2M belligerent and test solution.
|
Table 6: Impedance parameters for mild steel in 2M belligerent and test solution.
|
Conc. of Composite (ppm) |
Rs (Ω) |
Cdl (µ F cm-2) |
Rct (Ω cm2) |
Inhibition efficiency (%) |
Surface Coverage(θ) |
|
Blank |
0.6460 |
1196 |
0.3773 |
— |
— |
|
100 |
0.5787 |
772 |
0.5945 |
36 |
0.3656 |
|
200 |
0.6177 |
985 |
0.6168 |
40 |
0.3982 |
|
300 |
0.7320 |
722 |
0.7212 |
48 |
0.4768 |
|
400 |
0.6505 |
414 |
1.0076 |
62 |
0.6247 |
|
500 |
0.5549 |
594 |
1.0080 |
63 |
0.6269 |
The increase in Rct values have been assigned to the formation of protective layer at the metal/electrolyte interface.40 Decrease in Cdl values have been allotted to the increase in thickness of electrical double layer.41 Reflection of similar behavior in the present observation persist the inhibitive nature of composite.
Conclusion
Water soluble PANI coated aluminum oxide composite prepared exhibit good protection efficiency up to 88% in 1M acidic solution for two hours and is stable up to eight hours with little changes in efficiency (80%) on weight loss measurement. Increasing trend of corrosion resistivity upon increasing the concentration of composite is noticed in OCP measurements and electrochemical studies. All observations exposes that synthesized water soluble composite can equipped for industrial maintenance process.
Acknowledgement
The authors acknowledge the support from Dr.B.V., Dean for Research, SRM group of institutions, Dr. C. Jayaprabha, Associate Professor of Chemistry, University college of Engineering (Anna University) Dindigul-624622 and the Management of Rajalakshmi Engineering college, Chennai-600 105 for the electrochemical analysis of samples on free of cost. This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sectors.
References
- Wessling, B.; Passivation of metals by coating with polyaniline: Corrosion potential shift and morphological changes, Adv. Mater 1994, 6, 226-228.
- Cui, S.; Yin, X.; Yu, Q.; Liu, Y.; Wang, D.; Zhou, F. Polypyrrolenanowire/TiO2 nanotube nanocomposites as photoanodes for photocathodic protection of Ti substrate and 304 stainless steel under visible light Corros. Sci. 2015, 98, 471-477.
- Kewen Cai,; Shixiang Zuo,; Shipin Luo,; Chao Yao,; Wenjie Liu,; Jianfeng Ma,; Huihui Mao,; Zhongyu Li,; Preparation of polyaniline / graphine composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings RSC. Adv. 2016, 6, 95965
- Li, H.; wang, X.; Zhang, L.; Hou, B. Preparation and photocathodic protection performance of CdSe/reduced graphene oxide/TiO2 composite Corros. Sci. 2015, 94, 342-349.
- Sathiyanarayanan, S.; Syed Azim, S.; Venkatachari, G. Corrosion protection coating containing polyaniline glass flake composite for steel Electrochimica Acta 2008, 53, 2087–2094.
- Lebrini, M.; Lagrenée, M.; Traisnel, M.; Gengembre, L.; Vezin, H.; Bentiss, F.; Enhanced corrosion resistance of mild steel in normal sulfuric acid medium by 2,5-bis(n-thienyl)-1,3,4-thiadiazoles: Electrochemical, X-ray photoelectron spectroscopy and theoretical studies Applied Surface Science, 2007, 253, 9267-9276.
- Zhang, F.; Liu, J.; Li, X.; Guo, M.; Study of degradation of organic coatings in seawater by using EIS and AFM methods, J. Appl. Polym. Sci. 2008,109,1890-1899.
- Hongyang, G.; Wei, W.; Likun, X.; Li, M.; Zhangji, Y.; Xiangbo, L. Degradation behavior of a modified epoxy coating in simulated deep-sea environment J. Chin. Soc. Corrosion Protection 2017,37, 247–263.
- K.M. Deen, A.F.; Ahmad, R.; Khan, I.H.; Understanding the degradation mechanism of painted steel samples as a function of surface characteristics in artificial sea water Int. J. Chem. Mater. Sci. 2013,1, 013–021.
- Ashraf M.; El Saeed, M.; Abd El-Fattah, M.; Dardir, M.; Synthesis and characterization of titanium oxide nanotubes and its performance in epoxy nanocomposite coating Prog. Org. Coat. 2015, 78, 83-89.
- Zhang, X.Z.; Li, Y.J.; Effects of nano-sized titanium powder on the anti-corrosion property of epoxy coatings on steel, Kem. Ind. 2014,63, 317-322.
- Mishra, D.; Anand, S.; Panda 1, R.K.; Das, R.P. Hydrothermal preparation and characterization of boehmites, Materials Letters 2000,42,38-45.
- Laachachi, A.; Cochez, M.; Leroy, E.; Gaudon, P.; Ferriol1 M.; Lopez Cuesta, J. M. Effect of Al2O3 and TiO2 nanoparticles and APP on thermal stability and flame retardance of PMMA, Polym.Adv.Technol 2006, 17, 327-334
- Christophe Ne´dez,; Jean-Paul Boitiaux,; Charles J.; Cameron,; Blaise Didillon, Optimization of the Textural Characteristics of an Alumina To Capture Contaminants in Natural Gas, Langmuir, 1996, 12, 3927-3931.
- Zhanhu Guo,; Tony Pereira,; Oyoung Choi,; Ying Wang,; H. Thomas Hahn,; Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties, J. Mater. Chem. 2006, 16, 2800-2808.
- Alan G. MacDiarmid, Arthur J. Epstein, Polyanilines: a novel class of conducting polymers, Faraday Discuss. Chem. Soc., 1989,88, 317-332.
- Li, X. W.; Li, X. H.; Dai, N.; Wang, G. C.; Wang, Z. Preparation and electrochemical capacitance performances of super-hydrophilic conducting polyaniline, J. Power. Sources. 2010, 195, 5417-5421.
- Jiaxing Huang, Shabnam Virji, Bruce H. Weiller, Richard B. Kaner, Polyaniline Nanofibers: Facile Synthesis and Chemical Sensors, J. Am. Chem. Soc.2003, 125, 314-315
- Xia, H.; Wang, Q.; Ultrasonic Irradiation: A novel approach to prepare conductive polyaniline/nanocrystalline titanium oxide composites Chem. Mater 2002,14, 2158-2165
- Teoh, G. L.; Liew, K. Y.; Mahmood, Wan A. K. Preparation of polyaniline-Al2O3 composites nanofibers with controllable conductivity, Mater. Lett. 2007, 61, ,4947- 4949
- J. Zhu, S. Wei, L. Zhang, Y. Mao, J. Ryu, N. Haldolaarachchige, D.P. Young, Z. Guo, Electrical and dielectric properties of polyaniline-Al2O3 nanoparticles derived from various Al2O3 nanocomposite, J. Mater. Chem. 2011, 21, 3952–3959.
- Ahlatcioglu, E.; Celik Bozdogan, A.; Filiz Senkal, B.; Okutan, M.; The effect on the impedance characteristics of the metal oxide (Al2O3 and ZnO) doping in to polyaniline, Material Science in Semiconductor Processing 2016,56, 357-561.
- Kamatchi Selvaraj, P.; Sivakumar, S.; Selvaraj, S.; NiO-PANI composite as potential inhibitor for Mild Steel in acidic corrosion environment Int. J. chem. sci.2018 16(2) 268.
- Sivakumar, S.; Kamatchi Selvaraj, P.; Selvaraj, S.; Inhibition Efficiency of water Soluble Sr(ZnZr)1Fe10O19-PANI Composite against Strong acidic condition for Mild Steel, accepted for publication in Asian journal of chemistry,2018, 30 ISSN 0970-7077.
- Sivakumar, S.; Kamatchi Selvaraj, P.; Selvaraj, S.; Adsorption effect of simple metal oxide composite (CuO-PANI) on corrosion behavior of Mild Steel in low pH medium, Journal of Electrochemical Science and Technology 2018 under review.
- Majid Farahmandjou,; Nazafarin Golabiyan,; New pore structure of nano-alumina (Al2O3) prepared by sol gel method, Journal of Ceramic Processing Research. 2015 16 (2) 1-4.
- Manyasree D. A,; Kiranmayi P.; A, Ravi Kumar R.; V. S. S. N. B, Synthesis, Characterization And Antibacterial Activity Of Aluminium Oxide Nanoparticles, Int J Pharm Pharm Sci, 2018, 10,32-35.
- Sathiyanarayanan, S.; Syed Azim, S.; Venkatachari, G., A new corrosion protection coating with polyaniline-TiO2 composite for steel, Electrochimica Acta 2007,52,2068-2074.
- Yuetao Yi,; Guangyang Liu,; Zhining Jin,; Dawei Feng,; The use of conducting Polyaniline as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid, Int. J. Electrochem. Sci. 2013, 8, 3540-3550.
- Zhu, J.; Wei, S.; Zhang, L.; Mao, Y.; Ryu, J.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. Electrical and dielectric properties of polyaniline–Al2O3 nanocomposites derived from various Al2O3 nanostructures J. Mater. Chem. 2011, 21,3952-3959.
- Ahmed A. Ahmed Al-Dulaimi,; Shahrir Hashim,; Mohammed Ilyas Khan,; Corrosion Protection of Carbon Steel Using Polyaniline Composite with Aluminium Oxide, Pertanika J. Sci. & Technol. 2011, 19, 329-337.
- Tawfik A Saleh.; Vinod K Gupta,; Synthesis and Characterization of alumina nano- particles Polyamide membrane with enhanced flux rejection performance, separation and Purification Technology 2012, 89, 245-251.
- Shek, C.H.; Lai, J.K.L.; Transformation Evolution And Infrared Absorption Spectra Of Amorphous And Crystalline Nano-Al203 Powders, Nanostructure Mateds 1997, 8, 605-610.
- Xiaomin Cai,; Xiuguo Cui,; Lei Zu,; You Zhang,; Xing Gao,; Huihin Lian,; Yang Liu Xiaod ong wang, Ultra High Electrical Performance of Nano Nickel Oxide and Polyaniline Composite, Polymer. 2017;9:288.
- Ashish kumar Singh, Quraishi, M.A.; The effect of some bis-thiadiazole derivatives on the corrosion of mild steel in hydrocholoric acid, Corrosion Science 2010,52,1373-1385.
- Wessling, B.; Posdorfer, J.; Corrosion prevention with an organic metal (polyaniline): corrosion test results Electrochim Acta, 1999, 44,2139
- Yaser Jafari, Sayed Mehdi Ghoreishi, Mehdi Shabani-Nooshabadi, Electrochemical deposition and characterization of polyaniline-graphene nanocomposite films and its corrosion protection properties J. of Polym. Res. 2016, 23, 91.
- Jayaprabha, C.; Sathiyanarayanan, S.; Venkatachari, G.; Polyaniline as Corrosion inhibitior for iron in Acid Solutions, J. of Applied Surface Polymer Science 2005, 101, 2144-2153.
- Mansfeld, F.; Kendig, M. W.; Tsai, S.; Recording and Analysis of AC Impedance Datafor Corrosion Studies Corrosion 1982,38, 570-580.
- Yujie Qiang,; Shengtao Zhang,; Shenying Xu,; Wenpo L, Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution J. of Colloid and interface science 2016, 472, 52-59.
- Lukman Olasunkanmi,; Ime Bassey Obot,; Mwadham M Kabanda,; Eno E. Ebenso, Some Quinoxalin-6-yl derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Studies, J. Phys. Chem. 2015, C 119 (28) 16004-1601.

This work is licensed under a Creative Commons Attribution 4.0 International License.









