Effect of Different Current Density on Properties of Electrodeposited NiCoCr Thin Films
S. P. Meena1 and R. Ashokkumar2
and R. Ashokkumar2
1Research and Development Centre, Bharathiar University, Coimbatore-641 046, TamilNadu, India.
2PG and Research Department of Physics, Thiruvalluvar Govt.Arts College, Rasipuram-637 401, TamilNadu, India.
Corresponding Author E-mail: meenaphysics2012@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/3404023
Article Received on : 18-06-2018
Article Accepted on : 27-07-2018
Article Published : 31 Jul 2018
NiCoCr thin films were electroplated for different current density. FCC structured crystals were observed in XRD study. Coercive force and magnetization value exhibits soft magnetic nature. Electroplated NiCoCr films with electrolytic current density (2, 3, 4 and 5 mil.Amp/cm2) shows uniform deposition on the substrate. Cobalt composition was minimum as 21.31 wt% for current density 5 mA/cm2. The consistence of chromium and nickel increased while current density was improved. Thin films deposition with high current density shows low hysteresis loss and soft magnetic nature.The hardness of deposits increases when current density is increased.
KEYWORDS:Crystalline Size; Electrodeposition; Electrolytic Bath; SEM; X-ray Diffraction; VHN; VSM
Download this article as:| Copy the following to cite this article: Meena S. P, Ashokkumar R. Effect of Different Current Density on Properties of Electrodeposited NiCoCr Thin Films. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Meena S. P, Ashokkumar R. Effect of Different Current Density on Properties of Electrodeposited NiCoCr Thin Films. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47898 |
Introduction
There is a rising interest in NiCo thin films with chromium for innovative applications.1 NiCo thin films reveal magnetic, detecting and catalytic properties as an element of nickel and cobalt metals formed into spinel type structures. NiCo is a spinel compound which can give sufficient transmissivity from visible wavelength.2-5 Ni–Co alloy are important magnetic alloy materials which are largely and commercially used in industry due to its excessive saturation magnetization, high coercive force, excessive electric permeability and minimum eddy current loss .This NiCo alloy are utilized in specific applications for example organic and inorganic alloy electro synthesis, super capacitors, electro catalyst for anodic oxygen reaction, IR translucent electrodes for panel displays, switches, sensors and optical limiters.6-10 It was proven that NiCo alloy has interesting magnetic properties. NiCoCr alloy due to its soft magnetic character and its great mechanical properties, have revealed an incredible potential in MEMS, NEMS, recording devices, optical and sensors industries.11-14 In this investigation, the magnetic, structural, elemental composition and mechanical characterization of electro plated NiCoCr films have been studied with different current density.
Experimental Part
The NiCoCr composite films were electroplated on copper substrate for current density 2, 3, 4 and 5 mil.Amp/cm2. The processing time of electroplating was maintained as 15 min. In this experiment, steel and Cu plates performed as anode and cathode respectively with 7.5 cm x 1.5 cm. dimension.15-19 Electroplating of NiCoCr composite were achieved in electrolytic bath which consists Cr2 (So4)3 (10 g/l), (NH4)2SO4 (40 g/l) ,CoSo4 (15 g/l), C6H8O7(10 g/l) and NiSo4 (30 g/l). Solution pH is maintained as 6.0 in electrolytic solution by adding NH3 and electro chemical process was processed for current density 2,3,4 and 5 mil.Amp/cm2. The cathode plate was taken out from bath after the time 15 min. Analysis with scanning electron microscope and X-ray diffractometer provide surface and structural characters of thin films. Vickers hardness test provide micro hardness of films. The vibrating sample magnetometer provides magnetic properties of NiCoCr thin films.20-21
Results and Discussion
Composition of Electrodeposited Thin Films
From result, the cobalt compositions decrease as 37.91%, 32.15%, 27.95% and 21.31% for current density 2, 3, 4 and 5 mA/cm2 respectively. Nickel compositions increase as 48.79%, 52.80%, 55.42% and 60.16% for current density 2, 3, 4 and 5 mA/cm2 respectively. Chromium compositions are as 13.30%, 15.05%, 16.63% and 18.52% for current density 2, 3, 4 and 5 mA/cm2 respectively. EDS result indicates that the films received with high current density have high nickel content. The high cobalt content of 37.91wt% was received for low current density. EDS result shows that nickel content increases when improving current density. The weight content of chromium increases while increasing current density. Fig 1, 2 and 3 show the variation of cobalt, nickel and chromium composition with increasing current density.
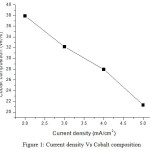 |
Figure 1: Current density Vs Cobalt composition. Click here to View figure |
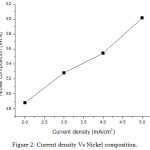 |
Figure 2: Current density Vs Nickel composition. |
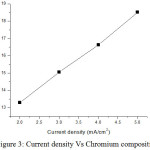 |
Figure 3: Current density Vs Chromium composition. |
Morphological Observation
The surface investigation of the Ni-Co-Cr magnetic films with various current density was observed by SEM and pictures are shown in figure 4. Electroplated magnetic films appears without cracks and uniform. They are bright in nature. It concludes that the deposition of materials on the substrate is uniform.
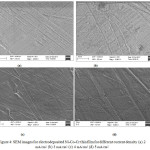 |
Figure 4: SEM images for electrodeposited Ni-Co-Cr thin film for different current density (a) 2 mA/cm2 (b) 3 mA/cm2 (c) 4 mA/cm2 (d) 5 mA/cm2 |
Structural Analysis
The crystal structure of Ni-Co-Cr alloy thin films was found by XRD analysis. Figure 5 demonstrates crystal patterns of the Ni-Co-Cr alloys which electrodeposited from electrolytic bath. The XRD studies indicates that electrodeposited magnetic alloys have different crystal orientations. XRD peaks of the alloys shows a preferred orientation along the (111), (200), (220), and (311) planes.
 |
Figure 5: XRD Patterns of Ni-Co-Cr thin films for different current density (a) 2 mA/cm2 (b) 3 mA/cm2 (c) 4 mA/cm2 (d) 5 mA/cm2 |
From XRD data’s, grain size values of the alloys on substrate were calculated using the FWHM (β),wavelength (λ) and Bragg’s angle(θ)
D=0.954λ/β cos θ
The average crystallite size is around 17 nm. The particle size decreases as 20.38, 18.13, 15.93 and 13.10 for current density 2, 3, 4 and 5 mA/cm2 respectively. Dislocation density and strain of Ni-Co-Cr alloy increase when current density increases. XRD data’s are shown in table 1. The crystalline size of thin film is 13.10 nm for current density is 5 mil.Amp/cm2.
Table 1: Structural characteristics of Ni-Co-Cr alloy thin films.
|
S.No |
Current Density (mA/cm2) |
2θ (deg) |
d (A0) |
Particle Size(D) (nm) |
Strain (10-3) |
Dislocation Density (1014 / m2) |
|
1 |
2 |
75.40 |
1.288 |
20.38 |
1.776 |
24.08 |
|
2 |
3 |
75.07 |
1.262 |
18.13 |
1.997 |
30.42 |
|
3 |
4 |
76.92 |
1.259 |
15.93 |
2.273 |
39.41 |
|
4 |
5 |
76.31 |
1.291 |
13.10 |
2.764 |
58.27 |
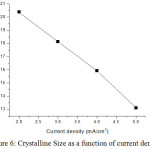 |
Figure 6: Crystalline Size as a function of current density. |
Mechanical Properties
Vickers hardness tester has given micro hardness of NiCoCr alloy on substrate. The micro hardness of alloy deposits with increasing current density are 54, 69, 76 and 95 VHN respectively. So it is confirmed that the hardness was improved while increasing current density. Fig 7 indicates the variation of hardness with increasing current density.
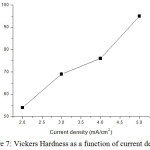 |
Figure 7: Vickers Hardness as a function of current density. |
Magnetic Properties of the Deposits
The magnetic hysteresis loops for Ni-Co-Cr alloy thin films with different current density are shown in figure 8. It is noted that magnetization value increases as 0.619 x 10-3 emu/cm2, 1.692 x 10-3 emu/cm2, 4.919 x 10-3 emu/cm2 and 10.04 x 10-3 emu/cm2 for current density 2,3,4 and 5 mA/cm2 respectively The film coated with high current density exhibits the low coercivity and high magnetization. The coercivity value decreases as 478G, 375G, 263G and 178 G for current density 2,3,4 and 5 mA/cm2 respectively. The retentivity values are 0.177 x 10-3 emu/cm2, 0.570 x 10-3 emu/cm2, 0.8174 x 10-3 emu/cm2 and 4.690 x 10-3 emu/cm2 for current density 2, 3, 4 and 5 mA/cm2 respectively. Squareness values are 0.2859, 0.3368, 0.1661 and 0.4668for current density 2, 3, 4 and 5 mA/cm2 respectively.
It was observed that the magnetization and retentivity increase by increasing current density. From VSM result, it is concluded that films prepared with high current density exhibits a higher value of saturation magnetization and low coercivity.
 |
Figure 8: Magnetic hysteresis loops for Ni-Co-Cr thin film for different current density (a) 2 mA/cm2 (b) 3 mA/cm2 (c) 4 mA/cm2 (d) 5 mA/cm2 |
Conclusion
The surface, elemental composition mechanical and magnetic characters of CoNiCr thin films have been investigated by varying current density (2, 3, 4 and 5 mA/cm2) at 30°C for 15 min. The thin films received with various current density are evenly coated and shiny. FCC structure was crystal formation of Ni-Co-Cr composite films. Micro hardness increases with increasing current density. When current density was improved from 2 to 5 mil. Amp/cm2, the crystallite size values decreases from 20.38 nm to 13.10 nm. This happens due to nano crystalline structure and low film strain associated with Ni-Co-Cr alloy thin films.When current density was improved from 2 to 5 mil.Amp/cm2, magnetization values increases from 0.619 x 10-3emu/cm2 to 10.04 x 10-3 emu/cm2. This happen due to nano structured crystal formation. So it concludes that soft magnetic character of NiCoCr is improved by increasing current density.
Acknowledgement
The authors wish to acknowledge Dr.T.Baskar, Professor, Dept. of S&H (Physics), Vidyaa Vikas College of Eng. and Technology, Tiruchengode, Tamilnadu, India for providing technical support.
References
- Gibbs, M.R.J.; Hill, E.W.; Wright, P.J. Jou. Phys. D: Appl. Phys. 2004, 37, 237-244.
CrossRef - Myung, N.V.; Park, D.Y.; Yoo, B.Y. ; Sumodjo, P.T.A. Jou. Magn. Magn. Mater. 2003, 265 , 189-198.
CrossRef - Yang Xiao-kui,; LI Qing,; Zhang Shi-yan,; GaoHui,; LuoFei,; Dai Yan. Journal of Solid State Electrochemistry 2010, 14(9) ,1601−1608.
CrossRef - JiangHui,; LI Yun-dong,; Huang Wei-hua,; TianHui. Applied Surface Science 2008, 254(21), 6865−6869.
- Emerson, R.N.; Kennady, C.J.;.Ganesan, S. Thin solid films 2007,515, 3391-3396.
CrossRef - Tury, B.; Radnóczi, G.Z.; Radnóczi, G.; Varsányi ,M .L. [J]. Surface and Coatings Technology 2007, 202(2), 331−335.
CrossRef - Shi, L.; Sun, C. F.; Zhou, F.;Liu, W. M. Material Science and Engineering A 2005, 397(1−2), 190−194.
CrossRef - Lupi, C.; Dell’era, A.; Pasquali, M.; Imperatori, P. [J]. Surface and Coatings Technology 2011, 205(23−24), 5394−5399.
CrossRef - Shi Lei; Sun Chu-feng; Gao Ping; Zhou Feng; Liu Wei-min. [J], Applied Surface Science 2006, 252(10), 3591−3599.
CrossRef - Chen, W.; Xia, C.; Alshareef, H.N. ACS nano. 2014, 9, 9531-9541.
CrossRef - Watson, S. W.; Walters, R. P. Journal of Electrochemical Society 1991, 138(12), 3633−3637.
CrossRef - Tury, B.; Lakatos, M.;Roya,S. Surface & Coatings Technology 2006, 6, 6713-6717.
CrossRef - Burzyn´ska, L.; Rudnik, E. [J]. Hydrometallurgy 2000, 54(2−3), 133−149.
CrossRef - Tian Liang-liang; Xu Jin-cheng; Qiang Cheng-wen. [J]. Applied Surface Science, 2011, 257(10), 4689−4694.
CrossRef - Fenineche, N. E.; Coddet, C.; Saida, A. [J]. Surface and Coating Technology, 1990, 41(1), 75−81.
CrossRef - Meena, S. P.; Ashokkumar, R. Rasayan J.Che. 2018,11 ,766-772.
CrossRef - QiaoGui-ying; Jing Tian-fu; Wang Nan; Gao Yu-wei; Zhao Xin; Zhou Ji-feng; Wang Wei. [J]. Electrochemica Acta 2005, 51(1), 85−92.
- Shi, Z.; Deng, K.; Li, L. Sci Rep. 2015,5,9317.
CrossRef - Ghaferi, Z.; Sharafi, S.; Bahrololoom, M.E. Appl. Surf. Sci. 2015, 355, 766-773.
CrossRef - Correia,A. N.; Machado,S.A.S. Electrochemica Acta, 2000 ,45, 1733-1740.
CrossRef - Ghaferi, Z.; Sharafi, S.; Bahrololoom, M.E. Appl. Surf. Sci. 2016, 375, 35-41.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









