Crown Ether Schiff bases and Their Complexes: Recent Advances Review
Khalid Maher and Shireen Mohammed
and Shireen Mohammed
Department of Chemistry, College of Science, Zakho University, Kurdistan, Region, Iraq.
Corresponding Author E-mail: maher-333@hotmail.de
DOI : http://dx.doi.org/10.13005/ojc/340402
Article Received on : 25-06-18
Article Accepted on : 17-07-2018
Article Published : 11 Aug 2018
In latterly years, an interest in the synthesis of crown ether Schiff bases and their Complexes has been increased, due to the significance and broadly the uses of these compounds in various fields. In the present review paper outline extensive recent advances literature survey on the crown ether including azomethine gathering and their complexes with the tough and easy granter atoms, has been reconsidered. Assertiveness has been done on element complexation with crown ether holding Schiff bases to enable the researchers to procure valuable information of the chelating activity of crown ether containing azomethine gathering and their enforcement.
KEYWORDS:Aza-Crown Ether; Complexes; Crown Ether; Ligands; Schiff Base
Download this article as:| Copy the following to cite this article: Maher K, Mohammed S. Crown Ether Schiff bases and Their Complexes: Recent Advances Review. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Maher K, Mohammed S. Crown Ether Schiff bases and Their Complexes: Recent Advances Review. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48220 |
Introduction
The first set of crown ethers compounds prepared by Pedersen assumes an essential part in the growth of supramolecular chemistry.1-4 In the area of supramolecular chemistry, crown ethers are suited to the gathering of most famous host group, since their implication complexes supply high practical applications.5-6 Macrocycles offer the thrilling potential to build supramolecular congregations that are fit for performing very particular atomic capacities. Molecular enforcement through these composition and their visitants, essentially transition elements ions or bio-molecules (nucleic acids, proteins, etc.), supply a high chance for seeking key side of supramolecular chemistry, which are also considered in a diversity of directions including, biology, medicine, physics, chemistry and related science and technology.7-9 The best notable character of crown ethers is their potential to obtain compounds electively with ionic hosts, such as cations of elements of the first group and the second group of periodic table, the various crown ether hole bulk can be elected to coordinate selectively with a given cation.10 Crown ether ligands with extra giver atom in the side arm have been prepared orderly to vary the ion bonding capability, sensitivity and selectivity of the origin crown ethers. Crown ethers11-13 along with Schiff base14-16 draw much consideration of coordination chemists. Collection of these fractions in one molecule superiority to am bis-dentate chelating systems able to figuration of both coordinate with d metals of intermediate hardness (Ni2+, Zn2+, Cu2+) coordinating via the azomethine part and crown ether complexes with toughs s-metal ions (Li+, K+, Na+, Ba2+).17,18 The coordination with d and s metals can be complete effectively in various arrangements to produce single- and di-nuclear compounds. The steadiness of the generate Macrocyclic metal buildings influenced basically by a few variables including the symmetry of the crown ether, the unit and sort of donners show in the ligand, variety and helpful impacts of neighbouring restricting destinations, relative size of the metal particle as for the crown ether hole estimate, the sign on the ion, adaptation of the ether cycle, the specific framework of the outlined ligands, their comparative location inside the macrocyclic shell, number and space of the coordinated cycle framed on complexation and the dissolvable, it is additionally conceivable to tailor-make distinctive kinds of atoms for particular uses, ion selective electrodes19 and in photosensitive frameworks.20 Crown ether holding azomethine gathering are famed to join base metal ions in the crown ether hole notwithstanding the chelating of a transition metal focus through the NO giver atom.21,22 The specific capacity of crown ethers to complex cations23 has been utilized to survey an extensive number of uses, for example, the creation of sensors and the particular removing of cations or the ionic transmit in velums.24 Crown ethers-containing Schiff bases are surveyed deficiently25-28. Since these ligands are imperative for the advancement of chemosensors and photograph triggers, and also for extraction of particles.1,2 Polyfunctional materials synthesized on the principle of metal buildings of azomethines29-32 are appeared by luminescent33-35 and magneto dynamic36,37 materials, catalysts,38 sensors,39-41 metal-containing polymers.42 In current years, there has been a major attention in the chemistry of antipyrine Schiff base crown ether.43-45 Crown ether azomethine gathering of 4-antipyrine and its buildings have an assortment of utilization in the natural, clinical, logical and pharmacological regions.46 Despite the fact that ambidentate ligands were analyzed in various investigations, just a single report has given an account of benzo-crown ether-4-antipyrine containing Schiff bases.47
Aza-Crown Ether Schiff Base
In latterly years an interest in the research of aza-Crown ethers containing azomethine gathering – metal complexes have been increased. Essentially, Crown ethers utilize as the substituents have encounter considerable interest, because of their coordinating capability to ions and specific arranging. Wei and his Co-Workers prepared some aza-crown ether holding single and di-azomethine groups (Scheme 1), orderly to examine the action of the aza-crown ring have stereo arranging an assignment on diver’s remarkable characteristic, namely, the strength for complexation with the metal ion and bio-mimetic performance.48
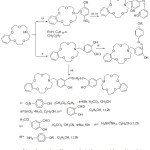 |
Scheme 1 |
Lu49 and Co-workers 2003 has been effectively prepared a range of Cobalt(II) complexes with aza-crown ether exhibit salicylaldimine azomethine moiety (Fig.1).
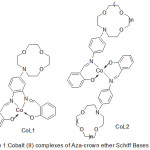 |
Figure 1 |
The cation chelating quality of the complexes and the steadiness constant with first and second metal cation have been studied. The synthesis of aza-crown ether Schiff Bases illustrated in (Scheme 2,3and 4).
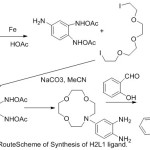 |
Scheme 2 |
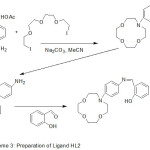 |
Scheme 3 |
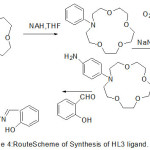 |
Scheme 4 |
Dioxygen consanguinity of azomethine Cobalt (II) Complexes with aza-crown ethers have been reported by Li50 and Co-Workers 2010. Set of aza-crown ether Schiff Bases were synthesized (Fig. 2). The result of the study shows that the existence of a pendant crown ether gathering in the imine ligand remarkably evolves the O2-binding abilities of the Complexes.
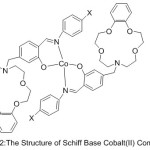 |
Figure 2 |
New salicylaldimine mono-Schiff bases with aza-crown pendant have been produced by condensing reaction of 3-[(benzo-10-aza-15-crown-methyl] salicylaldehye with substituted aniline (Scheme 5). The action of the coordination aza-crown ether moiety in the complexation with metal ions have been studied.51
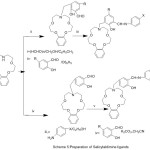 |
Scheme 5 |
Furthermore, work in Synthesis of N- (4-Salicylideneiminoaryl) mono aza Crown Ethers and Dioxygen consanguinity of their Cobalt (II) Compounds have been provided by Zeng 2003. The authors of this research designed and synthesized N-pivot lariat ethers with azomethine gathering as side branch, and predictable their cobalt (II) compounds to have enhanced dioxygen consanguinity52 (Scheme 6) and (Fig. 3).
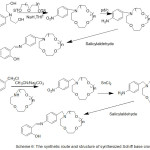 |
Scheme 6 |
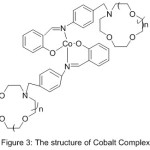 |
Figure 3 |
More searching about the preparation of Aza-crown Ether-substituted Mono-imine and Dioxygen consanguine of their Co (II) Complexes carried out by Wei 2004. The effect of the substituted aza-crown rings and their binding sites on dioxygen affinities of the Cobalt (II) complexes were discussed and compared with that of crown free analogues53 (Fig.4).
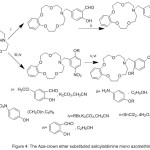 |
Figure 4 |
Novel Series of mono-azomethine gathering crown-ether and their metal compounds has been synthesized (Fig. 5), and their catalytic execution in PNPP hydrolysis, and the kinetics and the mechanism of PNPP catalytic hydrolysis in buffer solution have been investigated.54
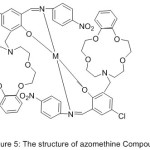 |
Figure 5 |
Cobalt (II) Salen Compounds with diaza-crown pendants were perfectly prepared beginning with benzo-10-aza-15-crown-5(Scheme 7) and (Fig.6).
The modification of O2-binding abilities by pendant substituents was studied, and contrast with the origin azomethine gathering compounds CoL1 (CoSalen). The outcome signalizes that the dioxygen consanguine of CoL has been highly promoted by aza-crown pendants compared with that by morpholino pendants, and the O2-linking abilities of CoL1 and CoL2 with aza-crown pendants will also be promoted by insert alkali metal cations.55
 |
Scheme 7 Click here to View scheme |
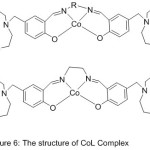 |
Figure 6 |
The complexation of a set of novel azomethine compounds holding the N-phenylaza-15-crown-5moiety with first and second groups of periodic table metal ions (incl. Be2+ and Mg2+), was prepared (Figure 7) by Antonov and their co-workers 2001. The location of the N-phenylaza-15-crown-5moiety in the ligand structure and the properties of the metal ion was studied.56
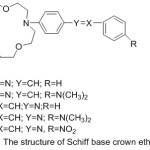 |
Figure 7 |
New uniform di-azomethine gathering Mn(III) and Co(II) compounds with benzo-10-aza-crown ether pendants (MnL1Cl, CoL1), and their similarities with morpholino pendants (MnL2Cl, CoL2), have been prepared and utilized as design to imitative hydrolase in p-nitrophenyl picolinate by Li and Co-workers 2006(Scheme 8).
 |
Scheme 8 |
The effect of temperature on the synthesized complexes signalizes that compounds are stead in the domain of the temperature studied in the work.57
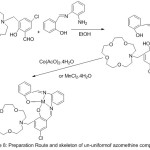
Khandar 2007 reported the preparation of Ni(II) compounds 16-membrane mixed-donor macrocyclic azomethine ligand, potentially hexa-dentate, holding two pendant alcohol groups (Scheme 9). The Ultra-Violet and Visible spectroscopy, conductivity determination and X-ray limitation show that the compounds are a distorted octahedral shape.58
 |
Scheme 9 |
Cupta 2010 reported the preparation of novel azomethine lariat ether binding based on 4,13-diaza-18-crown ether. The prepared ligand was the scout as a neuter ionophore for producing poly(vinyl chloride) based membrane sensors selective to silver59 (Fig. 8).
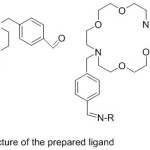 |
Figure 8 |
Condensing of 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene as a fluorophore connected at the 3-location to an N-thia macrocycle to an ethenylphenyl binder was provided by Isaad 2013 to obtain the water-soluble Chemosensor16 (Fig. 9).
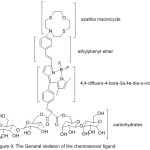 |
Figure 9 |
The synthesized Chemosensor was used as electro-electrode for uncovering Hg+2. Chemosensor 16 display noteworthy high ability to distinguish between Hg2+and structurally similar ions in coupling with a visible colorimetric and fluorometricchange.60
Aza-Crown Ether Schiff Base as Catalyst:
Zeng et al 2002reported the preparation of azomethine gathering holding aza-crown ether cycle and their transition elements compounds. The produced complexes were identified using different spectroscopy methods. The prepared Schiff bases were used as catalytic oxidation. They found that aromatic ring moiety in crown ether observed remarkably dioxygen affinities and biomimetic catalytic compared to uncrowned moiety61 (Scheme 10).
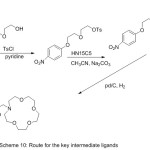 |
Scheme 10 |
More investigations about the synthesis of azomethine holding aza-crown ether cycles were provided. Zeng et al 2006reported the synthesis of salicylaldimine imine holding aza-crown ether cycle and their Mn (III) metal complexes (Fig. 10).
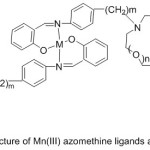 |
Figure 10 |
The prepared imine complexes were examined for their catalytic oxidation of styrene, they found that adding of the element of the first and the second group of periodic elements to the oxidation system. Increases the transformation of styrene to benzaldehyde62
A set of new unsymmetrical azomethine manganese (III) compounds with morpholino moiety have been prepared and investigated as catalysts in the respiratory oxidation of xylene to toluic acid (Scheme 11).
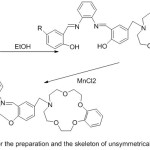 |
Scheme 11 |
The investigation illustrated that elective oxidation of para-xylene to para-toluic acid can effectively happen in the existing of azomethine Mn (III) complexes with pendant aza-crown group, which show a more best catalytic action than the azomethine Mn (III)compounds with pendant morpholino groupdo.63
Investigating the continuity of oxygenation and catalytic oxidation, Zeng et al 2004 reported the synthesis of mono-Schiff base holding crown ether cycles and their metal complexes (Fig. 11). The result of the study concluded that the azomethine compounds holding crown ether cycles effected on the dioxygen affinities and biomimetic catalytic oxidation.64
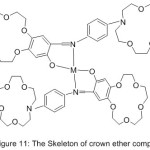 |
Figure 11 |
Li65 and his research team 2006, reported the synthesis of novel mono- azomethine cobalt complexes holding aza-crown moiety. The influence of Crown ether on the adjustment, of O2-coordinating ability and the catalytic oxidation of styrene were studied. The conclusion suggests that the dioxygen consanguine of the Cobalt complexes are too high performances by aza-crown pendants than that with morpholino pendants, and the O2-linking abilities of cobalt complexes with aza-crown pendants can also be promoted by inserting alkali metal cations (Fig. 12).
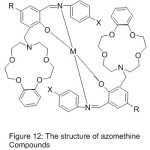 |
Figure 12 |
More works about the preparation of new mono azomethine Mn(III) Compounds holding aza-crown moiety have been provided by Yan et al 2006. The synthesized Complexes are used as the catalyst in the respiratory oxidation of 4-xylene to 4-toluic acid (Scheme 12). Result of the study indicates that in the inserting of alkali metal increases the transformation of 4-xylene to 4-toluic acid.66
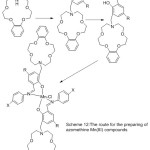 |
Scheme 12 |
A set of new aza-crown ether azomethine have been prepared and their host-guest complexation with C60, have been investigated in toluene using absorption spectroscopic method.67 All Produced compounds show stable complexes with 1:1 stoichiometry (Scheme 13).
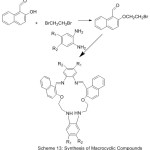 |
Scheme 13 |
New Complexes from Cobalt(II) and Manganese (III) with aza-crown ether containing Salen and Salophen substituents were prepared beginning from benzo-10-aza-15-crown-5(Fig. 13). The adjustment of O2-binding abilities and catalytic oxidation by these pendant substituent’s in the complexes were studied and resemble with the complexes ML5 and ML6. The results point out that the dioxygen consanguine eand catalytic oxidation affectivity of these complexes have been highly increased by aza-crown pendants than by morpholino pendants.68
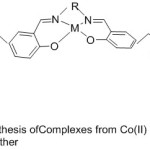 |
Figure 13 |
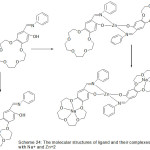
Sousa69 et al 2004, reported the synthesis of Ni (II) and Cu (II) complexes with azomethine holding aza-crown and two benzo-15-crown-5 moieties as elastic branches. The synthesized complexes were identified using different spectroscopies method (Scheme 14).
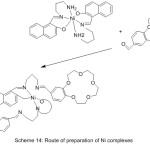 |
Scheme 14 |
Novel azomethine Mn(III) and cobalt(II) compounds with each of benzo-10-aza-crown ether pendants (MnL1, CoL1) or morpholino pendants (MnL2 2Cl, CoL22) have been utilize das patterns for hydrolase enzymes by mediate the kinetics of their hydrolysis reactions with p-nitrophenyl picolinate (PNPP)(Scheme 15)(Fig. 14).Result of the study described the effect of pH on the average of catalytic PNPP hydrolysis, which increased by increasing pH.70
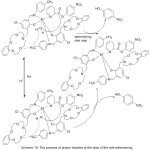 |
Scheme 15 Click here to View scheme |
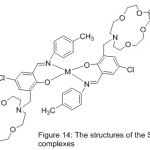 |
Figure 14 |
Novel mono-azomethine Mn(III) and cobalt(II) complexes with benzo-10-aza-crown ether pendants (MnL1, CoL1) and morpholino pendants (MnL2 2Cl, CoL22) were prepared and utilized as patterns for hydrolase enzymes to enhance the hydrolysis of p-nitrophenyl picolinate (PNPP) in the buffered CTAB solution (Fig. 15).The result of the study indicates that In differentiation, with the non-crowned compounds, the crowned azomethine compounds display higher catalytic effectiveness or enhance PNPP hydrolysis.71
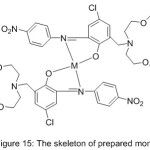 |
Figure 15 |
Hu et al 2010, illustrated the synthesis of two new Cobalt(II) compounds of the azomethine holding aza-crown moiety(Fig. 16). The prepared Schiff base complexes were investigated by the action of surfactant micelles on the activity of the hydrolysis of PNPP. The outcome appears that the azomethine Cobalt (II) compounds and their metallo-micelles as hydrolase imitate display useful catalytic effective and identical catalytic properties to normal enzyme.72
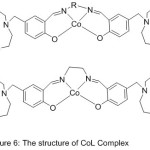 |
Figure 16 |
Novel Cobalt compounds with N- crown ether azomethine holding substituted salicylaldimine were prepared and utilized as design to imitate hydrolase in catalytic hydrolysis of the ester (Fig. 17).kinetic mechanism of PNPP hydrolysis have been studied. The kinetic mathematical design for PNPP cleavage catalyzed by the Cobalt compounds has been suggested. The outcome indicates that the bis(aza crown ether) Cobalt complexes show more activity in the PNPP catalytic hydrolysis.73
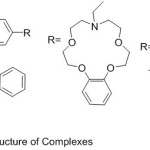 |
Figure 17 |
Crown ether containing azomethine gathering and their Metal Complexes
Novel Mn (II) azomethine complexes holding N-crown ether were prepared and identified using spectroscopy methods. The prepared complexes were used as catalysts for oxidation of cyclohexene and cyclo-octene by Oxone (Scheme 16). The action of crown-ether cycle connected to imine catalysts on the qualification of these catalysts was examined, the catalytic performance of these catalysts arises when an aromatic azo-base were added to the reaction.74
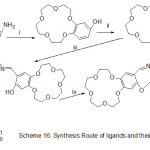 |
Scheme 16 Click here to View scheme |
Kandaz et al reported the synthesis of new soluble symmetrical phthalocyanine containing macrocycles (Scheme 17). The synthesized ligands were characterized using different spectroscopy methods. The author of these work achieved success in alkali-metal binding to the endo cyclic cap of crown ether by metal-ion.75
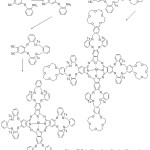 |
Scheme 17 |
Parkin and Menon76 reported novel crown ether azomethine synthesized from the reaction of different aldehydes with di-amino -18-crown-6. The Compounds which have been separated were purified and identified using various Spectroscopy method. All prepared Compounds appear liquid crystal properties.
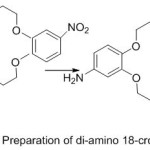 |
Figure 17a |
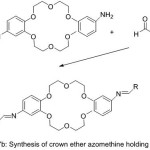 |
Figure 17b |
Zinc Complexes of Crown azomethine were synthesized and characterized using various Method of analysis (Scheme 18). The data of analysis indicate that in correspondence with the notion of hard and soft acids and bases, the hard lithium cation bonded to the crown moiety with the figuration of mono- (Li) and binuclear (Li, Zn) complexes.77
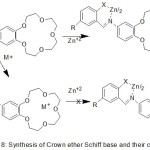 |
Scheme 18 Click here to View scheme |
Safin et al 2012, reported the preparation of 15-Crown-ether-exhibit N-salicylidene aniline compounds by reacting of 4-amino-15-crown-5 with salicylaldehyde (Fig. 18). The produced crown ether Schiff base was characterized using a different method of analysis. In addition of that, the optical properties of ligands were investigated.78
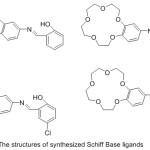 |
Figure 18 Click here to View figure |
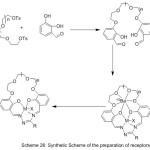
Jogani et al 2000, reported the preparation of a bi-branches crown ether possessed of two cation binding side branches containing an azomethine chelating derived from the reaction of crown ether, l8-crown-6 with aldehyde (Scheme 19) (Fig. 19). The complexes of azomethine with metal ions have been prepared and their chelating action wasinvestigated.79
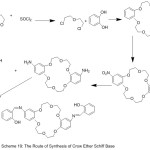 |
Scheme 19 |
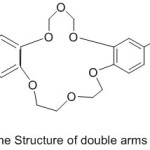 |
Figure 19 |
Azomethine ligands of 4-antipyrine and their complexes have an assortment of uses in the biological, medical, analytical and pharmacological areas. Although bi-dentate chelating compounds were investigated in many studies, only one of them has been reported on benzo crown ether-4-antipyrine holding azomethine gathering. Regarding this property, Hayvali,80 2009, reported the preparation of a novel crown antipyrine azomethine (Fig. 20).
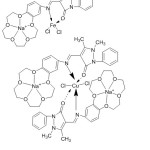 |
Figure 20 |
In the same way, Hayvali et al 2003, reported the synthesis of four novel-15-crown-5 ether ligands display different ortho-hydroxy Schiff base type horizontal function (Fig. 21). The synthesized Schiff base containing Crown ether moiety was characterized using different analysis methods.81
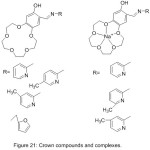 |
Figure 21 |
More work was done by Guler and Hayvali,82 they reported the preparation of novel benzyloxybenzaldehyde derivatives by the condensation of 4-benzonitrilewith vanillin, o-vanillin, 2-hydroxy-4-methoxy-benzaldehydeand 2-hydroxy-5-methoxybenzaldehyde (Scheme 20 and 21).
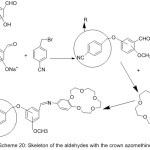 |
Scheme 20 |
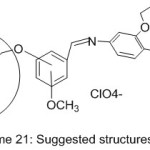 |
Scheme 21 Click here to View scheme |
Fernando and his team 2011, has been successfully prepared di-metallated Crown azomethine Palladacycles complexes (Fig. 22). They tried to use tetradentate [C,N,N,C] crown ether azomethine to prepare di-cyclo-metallated complexes having a polymeric Skeleton. Furthermore, the activity of such ligands toward tertiary mono- and diphosphines have been tested orderly to detect new, and full of hope unforeseen, results.83
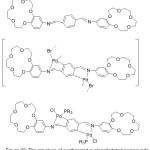 |
Figure 22 |
A novel set of poly Schiff base holding semi-hard big macrocycle in the major branched (bis -5-aldoxy-1,3-phenylene-32-crown -10) has been prepared in one pot of reaction.84 A direct poly condensation reaction of bis -5-aldoxy-1,3-phenylene-32-crown -10 in DMF produced a great Molecular weight poly crown azomethine compounds (Scheme 22)
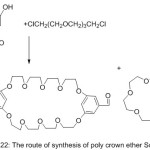 |
Scheme 22 |
N-(4′-benzo-15-crown-5-ether)-anthracene-9-imine as crown azomethine was synthesized by Bae85et al 2001and used it as a neutral transporter for ion electric-electrode to locate potassium by potentiometry (Fig. 23).
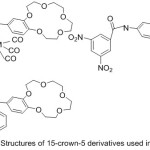 |
Figure 23 |
Four novels Mn (III) complexes with a crown holding salicylaldimine azomethine ligand have been successfully prepared86 and utilized as design to imitate hydrolase in the hydrolysis of 4-nitrophenylpicolinate (PNPP). The kinetics and mechanism of catalytic PNPP hydrolysis have been studied. The results signalize that the crown azomethine metal complexes appeared more catalytic power compared with complexes without crown ether Schiff base (Fig.24).
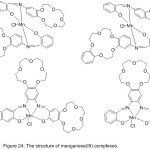 |
Figure 24 |
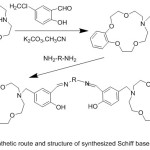
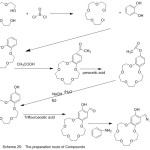
Condensation of 4-aminobenzo-15-crown-5 with aromatic aldehydes produced novel stable of crown ether azomethine ligands. Sadoveskaya87 et al 2015, synthesized novel ligands containing crown azomethine. The prepared ligands were identified using several analysis methods (Scheme 23).
 |
Scheme 23 |
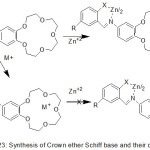
Novel crown ether azomethine from an N-salicylidenaniline compound with 15-crown-5 moiety, was prepared.88 Coordination of prepared ligands with some metal ion with Na+ or/and Zn+2 encourage evident changes in fluorescence spectra. Crown ether moiety after chelating with metal ion, the electron-rich metals in crown moiety will be coordinated. Hence, the PET action from crown giver across the fluorophore is closed and high fluorescence will be formed (Scheme 24 and 25).
 |
Scheme 24 |
 |
Scheme 25 Click here to View scheme |
Moutet89 et al 2001, synthesized novel ligands holding azomethine moiety as a redox center and a crown-ether-like segment as a chelating site. These complexes produced from matrix combine of bi compartmental macrocyclic compounds with one tough and one smooth gap, formed by condensation of asemicarbazide with a linear polyether aldehyde in the presence of metal cation (Scheme 26)
 |
Scheme 26 |
Conclusions
Aza-crown ether Schiff bases have been used widely in synthesis of chelating ligands. Crown ether containing azomethine groups are famed to join base metal ions in the crown ether hole notwithstanding the chelating of a transition metal focus through the NO giver atom. Crown ether ligands with extra giver atom in the side arm have been prepared orderly to vary the ion bonding capability, sensitivity and selectivity of the origin crown ethers. Crown ethers along with Schiff basedraw much consideration of coordination chemists. Collection of these fractions in one molecule superiority to am bis-dentate chelating systems able to figuration of both coordinate with d metals of intermediate hardness (Ni2+, Zn2+, Cu2+) coordinating via the azomethine part and crown ether complexes with toughs s-metal ions (Li+, K+, Na+, Ba2+).
References
- Steed, J. W.; Turner, D. R.; Wallace, K. J.; Core concepts in supramolecular chemistry and nanochemistry. Hoboken, NJ: John Wiley & Sons; 2007.
- Liu, Y.; Han, J.-R.; Zhang, H.-Y.; Supramol. Chem.2004, 16, 247.
CrossRef - Silverio, C.; Carlos, C.; Pablo, E.; Jean-Louis, G.; Daniel, G.; Bertrand, D.; Eur. J. Inorg. Chem.2008, 8, 1210.
- Faridbod, F.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P.; Riahi, S.; Sensors, 2008, 8, 1645.
CrossRef - Gokel, G.W.; Leevy, W.M.; Weber, M.E.; Chem. Rev.2004, 104, 2723.
CrossRef - Fery-Forgues, S.; Al-ali, F.; Photochem. J.; Photobiol. C: Photochem. Rev., 2004, 5, 139.
CrossRef - Chandra, S.; Gupta L.K.; Spectrochim. Acta,2004, 60A, 1563.
CrossRef - Boojar, M.M.A.; Shockravi, A.; Bioorg. Med. Chem.,2007, 15, 3437.
CrossRef - Liu Y.; Tetrahedron Lett., 2007, 48, 3871.
CrossRef - Castro-Juiz, S.; Andez, A. F.; opez-Torres, M. L; Azquez-Garcı´a, D. V.; Su_arez, A. J.; Vila, Jos_e M., and. Fern_andez, Jes_us J; Organometallics, 2009, 28, 6657.
CrossRef - Bren, V.A.; Usp. Khim., 2001, 70(12), 1152.
CrossRef - Nelsen, D. L.; Wheit, P. S. and Gagne, M. R.; Organometalics, 2005, 24, 5479-5453.
CrossRef - Tsivadze, A.Yu.; Usp. Khim., 2004, 73,1,6.
- Li, J. Z.; Xu, B. F., ZHOU F.M.; QIN, B., Yingb S.; Chinese Journal of Chemistry, 2007, 25, 1681—1685.
CrossRef - Hernandez-Molina, R. and Menderos, A.; Comprehensive Coordination Chemistry, McCleverty, J.A.A.and Meyer T.Y.; Eds., Amsterdam: Elsevier, 2003, 1, 411.
- Garnovskii, A.D. and Vasil’chenko, I.S.; Usp. Khim., 2005, 74, 3,211.
- Arion, V.B.; Kravtsov, V. Ch.; Gradinaru, J.I.; Simonov, Y.A.; Gerbeleu, N.V.; Lipkowski, J.; Wignacourt, J.-P.; Vezin, H. and Mentre,O.; Chim. Acta, 2002, 328,1,123.
- Uraev, A.I.; Nefedov, S.E.; Dorokhov, A.V.; Borisenko, R. N; Vasil’chenko, I.S.; Garnovskii, A. D and Tsivadze, A.Yu.; Ross. Khim. Zh., 2004, 48,1,38.
- Gloe, K.; Graubaum, H.; Wust, M.; Rambusch, T.; Seichter, W.; Coord. Chem. Rev., 2001, 222, 103.
CrossRef - Valeur, B.; Leray, I.; Coord. Chem. Rev. 2000, 205, 3.
CrossRef - Hayvali, Z.; Transition Met. Chem., 2009, 34, 97.
- Hayvali. Z.; Raman, N.; Thangaraja, C.; Johnsonraja S.; Cent Eur J Che.,2005,3(3), 537.
- Steed, J.W.; Coord. Chem. Rev., 2001, 215, 171.
CrossRef - (a) K. M. C.; Lu, X. X.; Cheung, K. K.; Zhu, N. Chem., Eur. J.2002, 8, 4066. (b) Breccia, P.; Van Gool, M.; Perez-Fern_andez R.; Martín-Santamaría, S.; Gago, F.; de Mendoza Prados, P.; J. J. Am. Chem. Soc. 2003, 125, 8270.
CrossRef - Tuntulani,T.; Poompradub, S.; Thavornyutikarn, P.; Jaiboon, N.; Ruangpornvisuti, V.; Chaichit, N.; Asfaric Z. and Vicensc, J.;Tetrahedron Letters, 2001, 42, 5541–5544
CrossRef - Siu, P. K. M.; Lai, S. W.; Lu, W.; Zhu, N.; Che, C. M. Eur. J. Inorg. Chem.2003, 2749.
CrossRef - Nakabayashi, T.; Ishida, T. and Nogami, T.; Inorg. Chem. Commun., 2004,7,1221.
CrossRef - Yoon, I.; Goto, M.; Shimizu, T.; Lee, S. S. and Asakawa, M.; J. Chem. Soc., Dalton Trans., 2004,10, 1513.
CrossRef - Garnovskii, A.D. and Vasil’chenko, I.S.; Usp. Khim.,2002, 71(11), 1064.
CrossRef - Breccia, P.; Van Gool, M.; Perez-Fernandez, R.; Martín-Santamaría, S.; Gago, F.; Prados, P.; de Mendoza, J. J. Am. Chem. Soc.2003, 125, 8270.
CrossRef - Garnovskii, A.D.; Vasilchenko, I.S.; Garnovskii, D.A.; and Kharisov, B.I; J. Coord. Chem., 2009, 62(2),151.
CrossRef - Garnovskii, A.D.; Sadimenko, A.P.; Vasilchenko, I.S.; Garnovskii, D.A.; Sennikova, E.V. and Minkin, V.I.; Adv. Heterocycl. Chem., 2009, 93, 291.
CrossRef - Metelitsa, A.V.; Burlov, A.S.; Bezuglyi, S.O.; Borod kina, I.G.; Bren, V.A.; Garnovskii, A.D. and Min- kin V.I.; Koord. Khim.,2006, 23(12), 894.
- You, Y. and Park, Y.; J. Chem. Soc., Dalton Trans., 2009, 1267.
CrossRef - Zhang, L.; Jiang, F.; Zhou, Y.; J. Coord. Chem., 2009, 62(9) 1476.
CrossRef - Burlov, A.S.; Uraev, A.I; Ikorskii, V.N.; Nikolaev- skii, S.A.; Koshchienko, Vasilchenko, Yu.V.; Garnovskii, I.S.; Vlasenko, D.A.; Zubavichus V.G.; Ya.V.; Divaeva, L.N; Borodkin. G.S. and Garnovskii, A.D.; Zh. Obshch. Khim.,2008, 78(6),1002.
- Garnovskii, A.D.; Vasilchenko, I.S.; Garnovskii, D.A.; Burlov, A.S. and Uraev, A.I. Ross. Khim. Zh., 2009, 53(1), 199.
- Gibson, V.C. and Spitzmesser, S.K.; Chem. Rev., 2003, 103(2), 283.
CrossRef - (a) Dillon, R. E. A.; Stern, C. L.; Shriver, D. F. Solid State Ionics, 2000, 133, 247.
CrossRef
(b) Yam, V. W. W.; Tang, R. P. L.; Wong, K. M. C.; Lu, X. X.; Cheung, K. K.; Zhu, N. Chem.; Eur. J.2002, 8, 4066.
CrossRef - Burgette and S.C. and Lippard, S.J.; Coord. Chem. Rev., 2001, 216–217, 334.
- Bren, V.A.; Dubonosov, A.D.; Tsukanov, A.V and Minkin, V.I.; Ross. Khim. Zh., 2009, 53(1), 42.
- Whittell, G.R. and Manners, J.; Adv. Mat., 2007, 19, 3439.
CrossRef - Raman, N.; Kulandaisamy, A.; Shunmugasundaran, A.; Transit Met Chem, 2001, 26,131.
CrossRef - Ismail, K. Z.; Transit Met Chem,2000, 25, 522.
CrossRef - Raman, N.; Kulandaisamy, A.; Thangarajan, C.; Transit Met Chem, 2004, 29, 129.
CrossRef - Agarwal, R. A.; Singh, L.; Sharma K.; Bioinorg,Chem Appl, 2006,1.
- Hayvali, Z.; Hayvali, M.; Kilic, Z.; Hokelek, T.; J Mol Struct, 2001, 597, 223.
CrossRef - Wei, X.; Mao, Z.; and Qin, S.; Synthetic Communications,2004, 34(7), 1237-1246.
CrossRef - Lu, X. X.; Qin, S. Y.; Zhou, Z. Y.; Yam V. W. W.; Inorganica Chimica Acta, 2003, 346, 49-56.
CrossRef - Li, J. Z.; Wei, L.; Feng, F. M.; Transition Met. Chem., 2010. 35, 463-468.
- Li, J.Z.; Yan, J.; Wei, X.; Zhou B. and Qin, S.Y.; Journal of Chemical Research, 2006, 7, 467-469.
CrossRef - Wei. Z.; Li, H. B.; Xiang R. li.; Wei, X.Y., Chinese Journal of Chemistry, 2003, 21, 510-514.
CrossRef - Wei, X.Y., Mao, Z. H.; Li, J. Z.; Qin S. Y.; Acta Chimica Sinica, 2004, 62(10), 969-974.
- Li, J. Z.; Lu, Y.; Wei, L.; Hu, W. and Qin, S. Y.; Progress in Reaction Kinetics and Mechanism.2010, 35, 368–386.
CrossRef - Wei, X.Y.; Li, J. Z.; Mao, Z. H.; Zhou B.; Qin S. Y.; Chinese Journal of Chemistry, 2004, 22, 558-562.
CrossRef - Antonova, L.; Vladimirova, M.; Stanoeva, E.; Fabian, W. M. F.; Balleste L. and Mitewa M.; Journal of Inclusion phenomena and Macrocyclic Chemistry, 2001, 40, 23-28.
CrossRef - Li, J. z.; Xu, B.; wang, y. and Li, S. X.; Transition Metal chemistry, 2006, 31, 487-494.
CrossRef - Khandar, a. A.; Yazdi, s. a. h.; Khatamian, M.; McArdle, P.; Zarei, A.; Polyhedron, 2007, 26, 33-38.
CrossRef - Cupta, V. k.; pal, m. j.; Singh, A. k.; Analytica Chimica Acta,2009, 631, 161-169.
- Isaad, j.; El Achari, A.; Analyst, 2013, 138, 3809-3819.
CrossRef - Zeng, wei.; mao, Z.; Wei, X.; Li, J.; Hong, Z. and Qin, S.; Journal of Supramolecular Chemistry,2002, 2, 501-507.
CrossRef - Zeng, W.; li, J.; Qin, S.; Inorganic Chemistry communications,2006, 9, 10-12.
CrossRef - Li, J. Z.; Wang, Y.; Zeng, W.; Qin, S. Y.; Supramolecular Chemistry,2008, 20(3), 249-254.
CrossRef - Zeng, W.; Li, J.; Mao, Z.; Hong, Z.; Qin, S.; Adv. Synth. Catal.,2004, 346, 1385-1391.
CrossRef - Li, J. Z.; Li, S. X.; Xie, F.; Transition Metal Chemistry, 2006, 31, 1066-1070.
CrossRef - Yan, j.; Li, J. Z.; and Li, Kc. B.; Transition Metal Chemistry, 2006,31, 286-292.
CrossRef - Mizyed S.; Kiwan R. and Marji D.; Jordan Journal of Chemistry, 2013, 8 (2), 71-78.
- Wei, X.; Li, J.; Zhou, B.; Qin, S.; Transition Metal Chemistry, 2004, 29, 457-462.
CrossRef - Sousa, C.; Gamerio, P.; Freire C.; De Castro, B,; Polyhedron,2004, 23, 1401-1408.
CrossRef - Li, J. Z.; Feng, F. M.’ Xu, B., Transition Metal Chemistry, 2008, 33, 655-660.
CrossRef - Li J. Z.; Feng F. M.; Zang J.; and Quin S. Y.; Journal of Dispersion Science and Technology,2011, 32, 14-22.
CrossRef - Hu W.; Li J.; Jin Z.; J.; Wang Y.; Zou L.; Du J.; and Wang X.; Journal of Dispersion Science and Technology, 2010, 31, 529–535.
CrossRef - Li J. Z.; Xie J. q.; Zeng W.; Wei X. Y.; Zhou B.; Zeng X. C.; and Qin S. Y.; Transition Metal Chemistry, 2004, 29: 488–494.
CrossRef - Seyedi S.M.; Gholam Hossein Zohuri G. H.; and Sandaroos R.; Supramolecular Chemistry, 2011, 23(7), 509–517.
CrossRef - Kandaz, M.; Yılmaz I.; Bekarog˘lu O.; Polyhedron, 2000, 19, 115-121.
CrossRef - Parikh V. B. And Menon, S. K.; Mol. Cryst.,2008, 482, 71-83.
CrossRef - Burlov, A.S.; Tsukanov, A.V.; Borodkin, G.S.; RevinskiiYu.V.; Dubonosov, A.D.; Bren, V.A.; Garnovskii A.D.; Tsivadze, A.Y.; Minkin, V.I.;Russian Journal of General Chemistry, 2006, 76(6), 992-996.
CrossRef - Safin D. A.; Robeyns K.; Garcia Y.; GrystEng Comm,2012, 14, 5523-5529.
- Jogani, S. K.; Menon S. K.; Agrawal, Y. K.; Synth. React. Inorg. Met.-Org. Chem, 2000, 30(7), 1317-1329.
CrossRef - Hayvali Z.; Transition Met. Chem.,2009, 34, 97-101.
- Hayvali Z.; Hayvali M.; Kilic Z.; Kokelek T. and Weber E.; Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2003, 45, 285–294.
CrossRef - Güler H.; Hayvali Z.; Dal H.; Hökelek T.; Polyhedrone, 2012, 31,688-696.
CrossRef - Fernandz A.; Margarita Lopez-Torres; Samuel Castro-Juiz; Manuel Merino; Digna Vazquez-Garcia; Josa M. Vila and Jesus J. Fernandez; Organometallics,2011, 30, 386–395
- Duta P. K.; Indian journal of Chemical Technology, 2001, 8, 515-517.
- Bae, Z. U; Lee S. B.; Chang S. H. and Kim U. R.; Journal of Korean chemical Society, 2001, 45(1), 31-39.
- Li J. Z.; Xu b. and Li S. X.; Transition Metal Chemistry, 2005, 30, 669-676.
CrossRef - Sadovskaya, N. Yu; Glushko, V. N.; Retivov, V. M.; Belus, S. K. and Grokhovskii V. V.; Russian Journal of General Chemistry, 2015, 85(12), 2771–2777.
CrossRef - Zhao L.; Chen X., Guo F., Gou B.; Yang C. and Xia W; Journal of Luminescence,2014, 145, 486-491.
CrossRef - Moutet J. C.; Aman E. S., U E. M.; arion V.; Gerbeleu N.; Revenco M.; Electrochimica Acta, 2001, 46, 2733-2740.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









