Biosorption of Cu (II) Metal Ions in Fixed Column by using Coconut Husk Waste
Harmiwati N.H , Khairul Akli, Rita Youfa, Maria Isfus Senjawati and Miftahul Khairati
, Khairul Akli, Rita Youfa, Maria Isfus Senjawati and Miftahul Khairati
Phytochemistry Department of Polytechnic ATI of Padang, Indonesia
Corresponding Author E-mail: harminahar@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/3404062
Article Received on : 27-03-2018
Article Accepted on : 23-06-2018
Article Published : 02 Aug 2018
A new technology has been currently developed for the removal of heavy metal waste in water, called biosorption. Biosorption technology has been widely used to remove heavy metals from liquid waste. The potential biomass which can be used as bio sorbent was activated coconut husk waste. By using the continuous flow method and activated coconut husk as bio sorbent, the obtained optimum flow rate and bed height of bio sorbent were 2 mL/min and 0.1 g with adsorption capacity of 188.322 mg/g.
KEYWORDS:Biosorption; Bio Sorbent; Fixed Bed Column; Heavy Metals
Download this article as:| Copy the following to cite this article: Harmiwati N. H, Akli K, Youfa R, Senjawati M. I, Khairati M. Biosorption of Cu (II) Metal Ions in Fixed Column by using Coconut Husk Waste. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Harmiwati N. H, Akli K, Youfa R, Senjawati M. I, Khairati M. Biosorption of Cu (II) Metal Ions in Fixed Column by using Coconut Husk Waste. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47982 |
Introduction
The current rapid industrial development has caused pollution effects on the environment. The activities of metal coating industry have resulted in the increasing of heavy metals level in the water because the waste is discharged into water.1,2 The heavy metals waste in aquatic environment can be dangerous for the living organism as it can lead to bio toxic effects, and cause the acute and chronic illness.3
Conventional technology has been widely used to remove heavy metals from liquid wastes such as chemical precipitation, filtration, ion exchange, coagulation, extraction, and reverse osmosis.4 Nevertheless, these methods are limited by technical and economic factors. The new technology has been currently developed for the removal of heavy metal waste in water. Biosorption is an adsorption process where the heavy metal waste in liquid is adsorbed by a solid adsorbent from biomaterial waste, called as bio sorbent. This technology has advantages in comparison with conventional technologies where the metal adsorption capacity is higher. Bio sorbent used from agricultural wastes is widely available in nature, thus providing more benefits compared to previous conventional techniques mentioned, which are cheaper, faster in adsorption, higher efficiency, and can be regenerated.5,6,7
Some biomass have been widely used as bio sorbents for heavy metals, such as wheat straw,8 peanut shells,9 Acacia leucocephala bark,10 banana peel,11 solid waste from olive oil production12 tobacco dust,13 rice husk,14 Moringa oleifera leaves,15 coconut shell,16,17 Annona muricataseeds,18 almond shell,19 oil palm shell,20 Nypa fruticans shell,21 Lansium domesticum fruit peel,22 Phaleria macrocarpa,23 soybean.24
One of the potential biomass which can be used as bio sorbent is activated coconut husk waste.
Coconut husk is a highly potential bio sorbent because it contains cellulose and lignin. Cellulose and lignin are biopolymers which play an important role in the separation process of heavy metals. Carboxyl groups of cellulose and phenolic acid of lignin take part in the bonding of metals. Hence, the use of coconut husk waste as bio sorbent to remove heavy metals from waste water especially in industrial waste water can be a promising potential. The novelty of this research was on the use of fixed column method for the biosorption of Cu (II) metal from industrial waste water using coconut husk waste as bio sorbent.
Material and Methods
Tools and Materials
The materials used in this research were coconut husk, metal solution of Cu(NO3)2, NaOH, HCl, HNO3, and aquadest. The equipment used in this research were glass column with diameter of 1 cm, length of 30 cm, peristaltic pump, scanning electron microscope with energy dispersive X-ray (SEM EDX), atomic adsorption spectrophotometer (AAS), fourier transformed infrared (FTIR), digital balance, pH meter, crusher, screening, paper strain, and glassware.
Bio sorbent Preparation
The bio sorbent raw materials, i.e. coconut husk was cleaned from sand and mud, washed with clean water, and dried in the open air. After dried, coconut husk was cut with size of 3 cm, then smoothed by using crusher with particle size of 425 μm. The pureed coconut husk was then activated by immersed in 0.01 M HNO3 solution for 2 hours while stirred occasionally. It was then washed with aquadest until the pH of 7. Activation was carried out to open the pores of the functional group, thus easily to be entered by metal ions, besides, it also aimed to remove contamination with other metal ions.
Coconut Husk Biosorbent Characterization
The characterization of coconut husk bio sorbent, i.e. FTIR and SEM EDX analysis, were done before and after adsorption.The analysis aimed to investigate the functional groups of bio sorbent which play an active role in the adsorption, changes in bio sorbent surface morphology before and after adsorption, and changes in the chemical composition of bio sorbent before and after adsorption.
 |
Figure 1: Coconut Husk (a) before treatment (b) bio sorbent |
Fixed Column (fixed bed column)
The study was conducted by continuous method using fixed bed column with initial concentration of 150 mg/L, solution pH of 4, bio sorbent mass of 0.1 g with bio sorbent particle size of 425 μm. The adsorption of heavy metal ions was investigated by passing a solution containing metal on a fixed bed column containing bio sorbent. The feeding solution was pumped into the column by varying the feed flow rate and the bio sorbent mass.
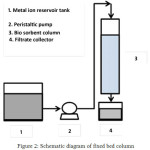 |
Figure 2: Schematic diagram of fixed bed column.
|
Results and Discussion
Biosorbent Characterization
FTIR Analysis
The result of FTIR analysis in Figure 3 and Figure 4 showthat there was a shift of wave number on OH and CH functional groups in coconut husk biomass before and after adsorption at several frequencies in FTIR analysis. The shift of wave numbers indicated the occurrence of metal ion binding on the OH and CH functional groups.25
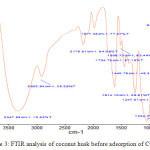 |
Figure 3: FTIR analysis of coconut husk before adsorption of Cu (II) ion. |
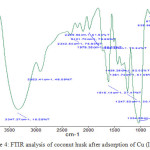 |
Figure 4: FTIR analysis of coconut husk after adsorption of Cu (II) ion.
|
SEM EDX Analysis
Variation of Flow Rate
The biosorption of Cu (II) metal ions was done by using the variation of flow rate of 2 ml/min; 4 mL/min and 6 mL/min, while other variations were kept constant, i.e. diameter of coconut husk biomass particles of 425 mm with mass of 0.1 g (1.5 cm) and metal ion concentration of 150 mg/L at pH = 4. The breakthrough curve for Cu (II) metal ion bio sorption was obtained from the relationship between the ratio of the filtrate concentration and the initial concentration of the metal ion (Ce/Co) against the time (t).
 |
Figure 5: Analysis of SEM EDX Coconut husk powder; (a) before adsorption; (b) after adsorption of Cu (II) ion. |
There was a significant change in the shape and gradient of the breakthrough curve on the variation of flow rate as shown in Figure 6. The higher flow rate can be seen by the lower slope of the breakthrough curve. On the breakthrough curve, it is also seen that the flow rates influence the saturation time of the Cu (II) metal ion biosorption. The higher flow rate resulted in the faster saturation time, but the adsorption capacity was not good at the high flow rate because the contact time/mass transfer zone between the metal ion and bio sorbent in the column was shorter. In this study, at the high flow rates, the columns were filled with many metal ions (flooding/channeling), thus the concentration of metal ions were not detected properly.26 This will be different when research was conducted at the low flow rates. At flow rate of 2 mL/min, qe = 188,794 mg/g, while at flow rate of 4 mL/min, qe = 183.917 mg/g and at flow rate of 6 mL/minute, qe = 130.665 mg/g. From the calculation, the best qe obtained was at flow rate of 2 mL/minute.
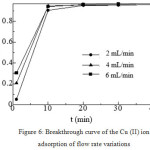 |
Figure 6: Breakthrough curve of the Cu (II) ion adsorption of flow rate variations. |
Bio sorbent Mass Variation
The effect of the variations of bio sorbent mass were examined. The bio sorbent mass/high bed (m) used were 0.1 g (1.5 cm); 0.15 g (2.25 cm); 0.2 g (3 cm); 0.25 g (3.75 cm); and 0.3 g (4.5 cm), while other variations were kept constant, i.e. metal ion concentration of 150 mg/L with pH of 4, diameter of bio sorbent particle of 425 mm, and metal ion flow rate of 2 mL/min. The breakthrough curve for Cu (II) metal ion biosorption was obtained from the relationship between the ratio of filtrate concentration and the initial concentration of metal ion against the time.
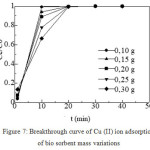 |
Figure 7: Breakthrough curve of Cu (II) ion adsorption of bio sorbent mass variations.
|
There was a significant change in the shape and gradient of the breakthrough curve for some of the bio sorbent mass variations used as shown in Figure 7. On the breakthrough curve, it can be seen that the bio sorbent mass influence the saturation time of the adsorption. The adsorption of Cu (II) metal ions in the column depends on the mass of the bio sorbent.
Breakthrough time was observed from the breakthrough curve as shown in Figure 7. The larger bio sorbent mass caused the slope of the breakthrough curve was lower than that of the smaller bio sorbent mass. This is due to the contact time/mass transfer zone of metal ions and bio sorbents in the column to achieve saturation was longer at the larger bio sorbent mass. Hence, as the mass of bio sorbent increased, it took longer time for the diffusion of metal ions into the pores of the bio sorbent. As indicated by its maximum adsorption capacity (qe), at the mass variation of 0.1 g, the obtained qe was 83.699 mg / g, while at the mass variation of 0.15; 0.2; 0.25; 0.3 g, the obtained qe were 55,799; 41,850; 33,480; and 27,900 mg/g, respectively. From the calculation, the larger bio sorbent mass resulted in the smaller adsorption capacity.27,8,28 The best adsorption capacity of Cu (II) metal ion was at bio sorbent mass of 0.1 g.
Conclusion
Coconut husk biomass waste can be a potential bio sorbent of Cu (II) metal ions in the waste water of metal industry. The optimum conditions of the continuous flow method of Cu (II) at flow rate of 2 mL/min and bio sorbent mass of 0.1 g with adsorption capacity of 188.794 mg/g.
Acknowledgements
This research was supported by the Ministry of Industry, Indonesia.
References
- Metcalf; Eddy. Mc Graw Hill Companies. 2003.
- Tiwari, M. K.; Bajpai, S.; Dewangan, U. K.; Tamrakar, R. K. Karbala International Journal of Modern Science. 2015, 1, 1–6.
CrossRef - SNI 7387. 2009.
- Ashraf, M. A.; Mahmood, K.; Wajid, A. International Conference on Food Engineering and Biotechnology IPCBEE. 2011, 9, 60–68.
- Amin, Y. Applied Biochemistry and Biotechnology. 2010, 160, 976–987.
CrossRef - Al-Haidary, A. M. A.; Zanganah, F. H. H.; Al-Azawi, S. R. F. Water, Air, and Soil Pollution. 2011, 214, 73–82.
CrossRef - Abdullah, Z.; Kurniawan, M. I.; Zein, R.; Aziz, H.; Munaf, E. Research Journal of Pharmaceutical Biological and Chemical Sciences. 2013, 4, 1443–1451.
- Doan, H. D.; Lohi, A.; Dang, V. B. H.; Dang-vu, T. Process Safety and Environment Protection. 2009, 6, 259–267.
- OuYang, X.K.; Yang, L.P.; Wen, Z.S. Bioresources Technology. 2014, 9, 2446-2458.
- Munagapati, V. S.; Yarramuthi, V.; Nadavala, S. K.; Alla, S. R.; Abburi, K. Chemical Engineering Journal. 2010, 157, 357–365.
CrossRef - Ashraf, M. A.; Wajid, A.; Mahmood, K.; Maah, M. J. Scientific Research and Essays. 2011, 6, 4055–4064.
CrossRef - Calero, M.; Bla, G. Journal of Chemical Engineering Data. 2011, 110, 3053–3060.
CrossRef - Qi, B. C.; Aldrich, C. Bioresources Technology. 2008, 99, 5595–5601
CrossRef - Zhang, Y.; Zheng, R.; Zhao, J.; Ma, F.; Zhang, Y.; Meng, Q. Biomed Research International. 2014, 2014, 1-8.
- Kumar, D. H.; Seshaiah, K.; Reddy, A. V. R.; Lee, S. M. Carbohydrate Polymers.2012, 88, 1077–1086.
CrossRef - Acheampong, M. A.; Pereira, J. P. C.; Meulepas, J. W.; Lens, P. N. L. Journal of ChemicalTechnology andBiotechnology. 2011, 86, 1184–1194.
- Acheampong, M. A.; Pakshirajan, K.; Annachhatre, A. P.; Lens, P. N. L. Journal of Industrial and Engineering Chemistry. 2013, 19, 841–848.
CrossRef - Fauzia, S.; Furqani, F.; Zein, R.; Munaf, E. Journal of Chemical and Pharmaceutical Research. 2015, 7, 573–582.
- Dionisio, E.; Bla, G.; Calero, M.; Ronda, A.; Martı, M. A. Journal of the Taiwan Institute of Chemical Engineers.2013, 44, 466–473.
CrossRef - Chong, H. L. H.; Chia, P. S.; Ahmad, M. N. Bioresource Technology. 2013, 130, 181–186.
CrossRef - Nazaruddin, N.; Zein, R.; Munaf, E.; Jin, J. Research Article. 2014, 6, 370–376.
- Furqoni, F.; Zein, R.; Munaf, E. Journal of Chemical and Pharmaceutical Research.2015, 7, 546–555.
- Nasution, A. N.; Amrina, Y.; Zein, R.; Aziz, H.; Munaf, E. Research Article. 2015, 7, 189–196.
- Harmiwati; Salmariza; Kurniawati, D.; Lestari, I.; Munaf, E.; Desmiarti, R.; Zein, R. Journal of Chemical and Pharmaceutical Research. 2015, 7, 94-100.
- Marin, A. B. P.; Aguilar, M. I.; Otruna, J. F.; Meseguer, V. F.; Saez, J.; Florenz, M. Journal of ChemicalTechnology andBiotechnology. 2010, 85, 1310 – 1318.
- Christoforidis, A. K.; Mitropoulos, A.C. Chemical Engineering Journal. 2015, 277, 334 – 340.
CrossRef - Mata, Y. N.; Munoz, J. A. Journal of Hazardous Material. 2009, 166, 612 – 618.
CrossRef - Luo, D.; Xie, Y. F.; Tan, Z. L.; Li, X. D. Journal of Environmental Biology. 2012, 34, 359 – 365.

This work is licensed under a Creative Commons Attribution 4.0 International License.









