Anticancer Activity of Graphene Oxide/5-FU on CT26 Ds-Red Adenocarcinoma Cell Line
Behrooz Afarideh1,4, Masoumeh Rajabibazl1,2 , Meisam Omidi3, Bahram Yaghmaee1, Azam Rahimpour2, Reza Khodabakhshi4 and Saeeideh Sarvarian4
, Meisam Omidi3, Bahram Yaghmaee1, Azam Rahimpour2, Reza Khodabakhshi4 and Saeeideh Sarvarian4
1Department of Clinical Biochemistry, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2Department of Tissue Engineering and Applied Cell Sciences, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3Protein Research Centre, Shahid Beheshti University, GC, Velenjak, Tehran, Iran.
4Fayazbakhsh Hospital, Social Security Organization of Iran, Tehran, Iran.
Corresponding Author E-mail: Rajabi_m@sbmu.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/3404038
Article Received on : 13-03-2018
Article Accepted on : 28-05-2018
Article Published : 16 Jul 2018
Cancer is one of the greatest health challenges in the world. Every year, many people die because of cancer. Chemotherapy is one of the treatment options in cancer disease. Fluorouracil )5-FU( is one of the chemotherapy dr0075gs, but it has relatively low toxic effect on tumor cells when it is used on free form, which also results in its poor efficacy. GO (graphene oxide) has a single-atomic layer and has several functional groups such as epoxide, carbonyl, carboxyl and hydroxyl which makes it a suitable carrier for drug loading. In the present study, we loaded 5-FU on GO nanocarrier to produce GO/5-FU, and characterized it by FT-IR. CT26 Ds-Red adenocarcinoma cell line was treated with GO/5-FU, free 5-FU, GO, and PBS (Phosphate buffer saline). The results showed significant inhibition of the CT26 Ds-Red cells using GO/5-FU compared to free 5-FU (P<0.05). Therefore, loaded 5-FU on GO (GO/5-FU) could be a new approach for optimization of 5-FU tumor cytotoxicity.
KEYWORDS:CT26 Ds-Red; Graphene Oxide; 5-FU
Download this article as:| Copy the following to cite this article: Afarideh B, Rajabibazl M, Omidi M, Yaghmaee B, Rahimpour A, Khodabakhshi R, Sarvarian S. Anticancer Activity of Graphene Oxide/5-FU on CT26 Ds-Red Adenocarcinoma Cell Line. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Afarideh B, Rajabibazl M, Omidi M, Yaghmaee B, Rahimpour A, Khodabakhshi R, Sarvarian S. Anticancer Activity of Graphene Oxide/5-FU on CT26 Ds-Red Adenocarcinoma Cell Line. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47376 |
Introduction
Cancer is one of the health-threatening diseases. In 2015, about 90.5 million people were affected by cancer.1 About 14.1 million new cases occur a year and it causes about 8.8 million deaths (15.7% of deaths).2 Fluorouracil (5-FU), is one of the choice drugs for chemotherapy of several cancers.3-5 5-FU acts in several ways, but principally as a thymidylate synthase (TS) inhibitor, synthesis of the thymidine, and the conversion of (dUMP) to (dTMP) which inhibits. Administration of 5-FU stops cancer cell dividing and causes cancer cell death.6-8 Loading of cancer drugs on nano-carriers is one of the ways to increase their therapeutic effect and biocompatibility. Graphene oxide is hydrophilic and monomolecular layer of graphite with various of functional groups such as epoxide, carbonyl, carboxyl and hydroxyl 9-13. The unique structural properties of GO has its wide application by many researchers as a nano-carrier to loading of several drugs. In prior studies 5-FU loaded on several nano-carriers, co-poly (d,l-lactic/glycolic acid) (PLGA) by Ywu-Jang Fu et al. 14, poly (HEMA) nanoparticles by Chouhan et al.,15 Pectin nanocarrier by Kumar Dutta et al.,16 polyamidoamine (PAMAM) by Ly, Uyen et al.,17 and poly(ε-caprolactone) nanoparticles (PCL NPs) by Ortiz et al.,18 Although previous studies had relative success to improve the antitumor activity of 5-FU by loading it on several nano-carriers, but more studies are need to improve the efficacy of 5-FU through loading it on new nano-carriers. The purpose of present study was the loading of 5-FU on GO to improve its antitumor activity. We loaded 5-FU on GO nano-carrier. New compound (GO/5-FU), free 5-FU, GO and PBS treated with CT26 Ds-Red adenocarcinoma cells and the results of GO/5-FU group compared with the results of 5-FU, GO and PBS treated groups.
Materials and Methods
Materials
5-FU was provided from Ebewe Pharma Co. The CT26 Ds-Red colon adenocarcinoma cell line purchased from Pasture Institute of Iran, then they were cultured in RPMI 1640 medium supplemented with L-glutamine (Biowest), 10% fetal bovine serum and penicillin-streptomycin (100 U/ml and 100 mg/ml, respectively) at 37°C in a 5% CO2 humidified atmosphere. Graphite powder was purchased from Merck. Other materials provided from local suppliers.
Methods
Synthesis of graphene oxide
Graphene oxide (GO) was synthesized by Hammer modified method.19 Briefly, graphite powder (3g) was oxidized in 12 ml of H2SO4 at the presence of 2.5 g of K2S2O8 and 2.5g of P2O5 at 80°C for 4.5 hours. Then the graphite oxide was neutralized using 120 ml of precooled sulfuric acid. KMnO4 (15g) progressively added into the solution under vigorous stirring under 20°C. Temperature was raised to 40°C and continued stirring for 2 h, Two times deionized water 250 ml, 700 ml and 20 ml of H2O2 was added under strong stirring, respectively. For elimination of excess materials, the mixture was washed and neutralized with 100 ml of HCl (37%), HCl aqueous solution 100 ml (1: 10 v/v), and deionized water (1000 ml), respectively. Neutralized graphene oxide was dried at the ambient temperature, dispersed (0.5% wt) in deionized water, and dialyzed with dialysis bag (7000 Da) for one week.
5-FU loading and its characterization
5-FU (3000 µg/ml) was added to GO suspension (1 mg GO/1ml of 100mM PBS) and incubated on darkness condition at 37°C for 24 h while slightly shaking (50 rpm).20 Unloaded 5-FU was removed by centrifugation (17740 RCF, 30 min, Eppendorf 5417 R) and three times washed with PBS (100 mM, PH 7.4).
Cells culture and MTT assay
CT26 Ds-Red colon adenocarcinoma cell line was cultured in RPMI1640 medium supplemented with L-glutamine (Biowest), fetal bovine serum 10% and penicillin-streptomycin (100 U/ml and 100 mg/ml, respectively) at 37°C, 5% CO2 and humidified atmosphere. MTT assay applied to measure the cytotoxicity of (GO/5-FU), free 5-FU, GO and PBS.21, 22 CT26 Ds-Red cells seeded and incubated overnight at 37°C, 5% CO2 humidified atmosphere, then the cells were treated by GO/5-FU, free 5-FU, GO and PBS. For each groups we considered four well (quadruplicate). After 24h incubation at 37°C, 5% CO2 and humidified atmosphere, 20 µl MTT solution was added and incubated for 4 hours at 37°C, then medium and MTT were removed. DMSO (200 µl) was added to all wells. Incubation was done for 15-minute in ambient temperature and darkness. Absorbance was measured by a microplate reader (Anthos 2020, 540 nm). To calculate the cell viability (%) the following formula was used23:
Cell viability (%) = (mean absorbance of treated cells/ mean absorbance of control cells) × 100
Statistical analyses
Data were analyzed with Graphpad Prism 6. Student’s t-test was used for analysis of the results between two groups. Data was presented as mean ± standard deviation (SD). Results were considered statistically significant at P < 0.05.
Results and Discussion
Synthesis and characterization of GO
Graphite used for synthesis of GO according of modified Hummers’ method.10 Obtained GO was characterized by atomic force microscopy (AFM), transition electron microscopy (TEM), X-ray powder diffraction (XRD) and FTIR spectroscopy methods to detect the shape, size, and chemical structure of obtained GO24 (Fig. 1).
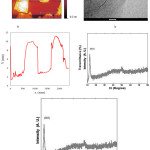 |
Figure 1a: AFM (atomic force microscopy)image and height profile of GO, (b)TEM (transition electron microscopy) of GO, (c) FTIR of GO , (−OH) and (C=O) spectrums of GO carboxyl group, were appeared at (3374 cm-1) and (1710 cm-1 ), respectively. (d) X-ray powder diffraction (XRD) patterns of GO. |
Drug loading and Characterization
5-FU loaded on GO nano-carrier according as described in materials and methods. Loaded 5-FU calculated by UV-vis spectrophotometry (CECIL CE 2041, 265 nm). 5-FU standard curve provided according to calculated absorbance for serial dilutions of 5-FU (Table 1, Fig. 2). Loaded 5-FU concentration was calculated according the following formula:
Table 1: Calculated absorbance for serial dilution of 5-FU by UV-vis spectrophotometry at 265 nm.
| 5-FU Concentration (µg/ml) | Absorbance |
| 0 | 0 |
| 3.75 | 0.145 |
| 7.5 | 0.29 |
| 15.5 | 0.565 |
| 31 | 1.052 |
| 63 | 1.71 |
| 125 | 1.98 |
| 250 | 2.3 |
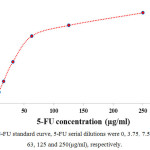 |
Figure 2: 5-FU standard curve, 5-FU serial dilutions were 0, 3.75, 7.5, 15.5, 31, 63, 125 and 250(µg/ml), respectively. |
Loaded 5-FU = (initial 5-FU concentration –5-FU concentration in supernatant)
The concentration of 5-FU on supernatant was 1254 (µg/ml) and initial concentration of 5-FU was 3000 (µg/ml). Therefore, in our study the concentration of loaded 5-FU on GO nano-carrier was 1746 (µg/ml). Dilution was performed for high concentration samples.
Cytotoxic activity of GO/5-FU
MTT assay was used to detect the cytotoxicity effect of GO/5-FU. We assayed GO/5-FU, free 5-FU, GO and PBS (100mM) on CT26 Ds-Red adenocarcinoma cell line, the results are shown in (Fig. 3, Table 2). The inhibition of CT26 Ds-Red cell line by GO/5-FU was significantly higher compared to free 5-FU (P<0.017) (Fig. 3).
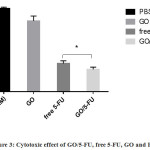 |
Figure 3: Cytotoxic effect of GO/5-FU, free 5-FU, GO and PBS. |
MTT assay was used to assay of cytotoxic effect (see material and method). Data presented as mean ± SD. Inhibition of CT26 Ds-Red cell by GO/5-FU was significant, (P<0.017).
GO/5-FU IC50
IC50 is a drug concentration which inhibit the growth of 50% cells.25 Calculated IC50 in present study for GO/5-FU and free 5-FU was 5.2 µg ml-1 and 8.1 µg ml-1, respectively. These results showed the IC50 of GO/5-FU was 35.8% lower compared to free 5-FU. In the other words, inhibition potency of GO/5-FU was higher (35.8%) in comparison with free 5-FU (Table 2).
Table 2: Calculated IC50 for GO/5-FU, free 5-FU and GO. Samples IC50 (µg ml-1)
| Samples | IC50 (µg ml-1) |
| GO/5-FU | 5.2* |
| 5-FU | 8.1 |
| GO | 28.9 |
The IC50 of GO/5-FU significantly was lower in compare free 5-FU (*P<0.05)
Loading of anticancer drugs on several nano-carriers is a new concept on chemotherapy and in the recent years, extensive research has been conducted on this subject. Fluorouracil (5-FU) is a common anticancer drug which is used in several cancers but its efficacy is low. The purpose of present study was improving the efficacy of 5-FU by loading it on GO nano-carrier. Our results showed that loaded 5-FU on GO (GO/5-FU) significantly inhibited CT26 Ds-Red adenocarcinoma cell line compared to its free form (5-FU). This improvement may be due to slow and steady release of 5-FU when it loaded on GO nano-carrier. The results are in agreement with those of TU et al.,17 NQ et al.,26 El-Ghannam et al.,27 Ganguly et al.28 and Liu et al.,29 However, our findings do not support those of Wang et al. study,30 in that, they found the amount of loaded 5-FU on KGM/SA/GO nano-carrier was 32% while in our study it was 58%. One possible explanation for this discrepancy is that our incubation condition (24 h, 37°C, shaking 50 RPM vs 24 h, room temperature and without any shaking) or it may be related to the nano-carriers differences (GO vs KGM/SA/GO). Our results regarding cell viability do not support those of Rana et al. study31 (26% vs 80%, respectively). This difference may be due to the application of different cancer cell lines (CT26 Ds-Red vs CEM, respectively). The results of present study showed the potential of GO/5-FU as novel compound for the enhancement of anti-cancer activity of 5-FU.
Acknowledgment
This article has been extracted from the thesis written by Mr. Behrooz Afarideh in School of Medicine Shahid Beheshti University of Medical Sciences, (Registration No: 424). The present study has been financially supported by Research Department of the School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant No. 8861).
References
- Vos, T.; Allen, C.; Arora, M.; Barber, R.; Bhutta, Z.; Brown, A.; Carter, A.; Casey, D.; Charlson, F., and Chen, A. 2016, 388, 1545-1602.
- Feigin, V. The lancet. 2016, 388, 1459-1544.
CrossRef - Simon, N.; Vasseur, M.; Pinturaud, M.; Soichot, M.; Richeval, C.; Humbert, L.; Lebecque, M.; Sidikou, O.; Barthelemy, C., and Bonnabry, P. PloS one. 2016, 11, e0159052.
CrossRef - Thelwall, M.; Kousha, K., and Abdoli, M. Scientometrics. 2017, 112, 509-527.
CrossRef - Wu, C. Surg. Oncol. Clin. N. Am. 2018, 27, 235-242.
CrossRef - Longley, D. B.; Harkin, D. P., and Johnston, P. G. Nat. Rev. Cancer. 2003, 3, 330-338.
CrossRef - Joag, M. G.; Sise, A.; Murillo, J. C.; Sayed-Ahmed, I. O.; Wong, J. R.; Mercado, C.; Galor, A., and Karp, C. L. Ophthalmology. 2016, 123, 1442-1448.
CrossRef - Álvarez, P.; Marchal, J. A.; Boulaiz, H.; Carrillo, E.; Vélez, C.; Rodríguez-Serrano, F.; Melguizo, C.; Prados, J.; Madeddu, R., and Aranega, A. Expert. Opin. Ther. Pat. 2012, 22, 107-123.
- Compton, O. C. and Nguyen, S. T. Small. 2010, 6, 711-723.
CrossRef - Hummers Jr, W. S. and Offeman, R. E. J. Am. Chem. Soc. 1958, 80, 1339-1339.
CrossRef - Wei, X.; Mao, L.; Soler-Crespo, R. A.; Paci, J. T.; Huang, J.; Nguyen, S. T., and Espinosa, H. D. Nat. Commun.. 2015, 6, 8026.
CrossRef - Wei, X.; Mao, L.; Soler-Crespo, R. A.; Paci, J. T.; Huang, J.; Nguyen, S. T., and Espinosa, H. D. Nat. Commun.. 2017, 8, 14488.
CrossRef - Yamada, Y.; Yasuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T., and Sato, S. J. Mater. Sci. 2013, 48, 8171-8198.
CrossRef - Fu, Y.-J.; Shyu, S.-S.; Su, F.-H., and Yu, P.-C. Colloids Surf. B. 2002, 25, 269-279.
CrossRef - Chouhan, R. and Bajpai, A. J. Mater. Sci. Mater. Med. 2009, 20, 1103-1114.
CrossRef - Dutta, R. K. and Sahu, S. Eur. J. Pharm. Biopharm. 2012, 82, 58-65.
CrossRef - Ly, T. U.; Tran, N. Q.; Hoang, T. K. D.; Phan, K. N.; Truong, H. N., and Nguyen, C. K. J. Biomed. Nanotechnol. 2013, 9, 213-220.
CrossRef - Ortiz, R.; Prados, J.; Melguizo, C.; Arias, J. L.; Ruiz, M. A.; Alvarez, P. J.; Caba, O.; Luque, R.; Segura, A., and Aránega, A. Int. J. Nanomed. 2012, 7, 95.
- Xu, Y.; Bai, H.; Lu, G.; Li, C., and Shi, G. J. Am. Chem. Soc. 2008, 130, 5856-5857.
CrossRef - Chen, L.; She, X.; Wang, T.; He, L.; Shigdar, S.; Duan, W., and Kong, L. Nanoscale. 2015, 7, 14080-14092.
CrossRef - Berridge MV; Herst PM , and and Tan AS 2005, 11, 127-152.
- Mosmann, T. J. Immunol. Methods. 1983, 65, 55-63.
CrossRef - Luciani, A.; Coccoli, V.; Orsi, S.; Ambrosio, L., and Netti, P. A. Biomaterials. 2008, 29, 4800-4807.
CrossRef - Hashemi, M.; Yadegari, A.; Yazdanpanah, G.; Jabbehdari, S.; Omidi, M., and Tayebi, L. RSC Advances. 2016, 6, 74072-74084.
CrossRef - Pei, y.; Feng Yang, F.; Chen, X.; Wu, N.; Wang, K, Chem. Eng., 2017, 5,3223–3232.
- Tran, N. Q.; Nguyen, C. K., and Nguyen, T. P. Adv. Nat. Sci.: Nanosci. Nanotech. 2013, 4, 045013.
- El-Ghannam, A.; Ricci, K.; Malkawi, A.; Jahed, K.; Vedantham, K.; Wyan, H.; Allen, L. D., and Dréau, D. J. Mater. Sci. Mater. Med. 2010, 21, 2701-2710.
CrossRef - Ganguly, K.; Kulkarni, A. R., and Aminabhavi, T. M. Drug delivery. 20151-14.
- Liu, W.; Li, X.; Wong, Y.-S.; Zheng, W.; Zhang, Y.; Cao, W., and Chen, T. ACS nano. 2012, 6, 6578-6591.
CrossRef - Wang, J.; Liu, C.; Shuai, Y.; Cui, X., and Nie, L. Colloids Surf. B. 2014, 113, 223-229.
CrossRef - Rana, V. K.; Choi, M. C.; Kong, J. Y.; Kim, G. Y.; Kim, M. J.; Kim, S. H.; Mishra, S.; Singh, R. P., and Ha, C. S. Macromol. Mater. Eng. 2011, 296, 131-140.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









