A Study of the Effects of Waste Egg and Shrimp Shells on the Toxicity Immobilisation of Chemicals
Department of Chemistry Faculty of Science and Technology, RMUTT Pathum Thani, Thailand
Corresponding Author E-mail: boontida_u@rmutt.ac.th
DOI : http://dx.doi.org/10.13005/ojc/3404028
Article Received on : 26-05-2018
Article Accepted on : 20-07-2018
Article Published : 27 Aug 2018
Natural resources are declining rapidly. Water scarcity is one of the biggest crises that the world faces. Even though there are many treatment methods, it still leaves a large carbon footprint. Hence, biological treatment is desired. However, there are many toxic compounds that contaminate wastewater, which inhibit biological treatment. Therefore, waste egg and shrimp shells were introduced as adsorbents to immobilize toxicity as a pre-treatment method. Egg and shrimp shells were tested for their surface charge with zeta potential. Then, Escherichia coli (E.coli) cells were exposed to the toxic compounds, copper sulfate and carbonyl phenol, for 15 minutes. Subsequently, E.coli cells were regrown on the agar plates to determine the recuperation rate of the colonies. It was found that eggshells decreased the toxicity of the tried concoction to E.coli, and enabled the colonies to recover by up to 62%. Moreover, it has proved that there is potential that the surface charge has an effect on the adsorption process. Eggshells, which have -21.85 zeta potential, adsorb copper sulfate, which is positively charged, better than carbonyl phenol, which is negatively charged; and vice versa for shrimp shells.
KEYWORDS:adsorption; toxicity immobilisation; waste materials; zeta potential.
Download this article as:| Copy the following to cite this article: Uapipatanakul B. A Study of the Effects of Waste Egg and Shrimp Shells on the Toxicity Immobilisation of Chemicals. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Uapipatanakul B. A Study of the Effects of Waste Egg and Shrimp Shells on the Toxicity Immobilisation of Chemicals. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48776 |
Introduction
Global environmental concerns are growing intensely with such a sharp decline in natural resources, pollution, and climate change. Moreover, with the continuous increase in world population1 has caused serious resource scarcity such as water. Water is an important resource for life and now, our waterways are polluted with chemicals from industry, agriculture, and household activities.
There are several wastewater treatment methods such as reverse osmosis,2 vacuum evaporation,3 ultrafiltration (UF),4 and chemical oxidation.5 These strategies are costly and resource intensive. Natural treatment is an exceptionally appealing treatment technique as it requires less energy and is sustainable. It avoids utilizing excess additional chemical compound,6 which means financially feasible and environmentally friendly. However, wastewater is often contaminated with harmful chemicals, for example, biocide from agricultural activities, and toxic chemical additives from the manufacturing; which biological treatment is impractical. Hence, toxicity immobilisation is necessary as a pre–treatment to remove toxins.7,8 and achieve successful biological treatment.
 |
Figure 1: The chart shows that hybrid technology of toxicity immobilisation can aid biological treatment. |
To immobilise toxicity, one of the popular methods is adsorption. Adsorption is the mechanism where particles or atoms of either gas, fluid, or solid attached to a surface of adsorbent. There are many materials that could be employed as an adsorbent such as clay,9 zeolite,10 and nano-iron.11 Natural adsorbents that are also well-acknowledged are, for instance, kaffir lime leaves,12 and rice husk.13 According to the green technology principle, the objective of this research was to employ natural waste materials, egg and shrimp shells as adsorbents and evaluated the ability to immobilise toxicity. This would not only act as hybrid technology and facilitate biological treatment of the wastewater, but also make the most of natural materials. In addition, in this work was aim to observe the relationship of surface charge to the adsorption ability as an another dimension of understanding.
Materials and Methods
Sample Preparation
Copper sulfate and carbonyl phenol were selected as candidates for toxic compounds, where copper sulfate represents negatively charged toxic compounds and carbonyl phenol represents positively charged toxic compounds. A dilution series of both samples were prepared. Samples were diluted in distilled water to 7 concentrations; 0.005% v/v, 0.01% v/v, 0.05% v/v, 0.1% v/v, 0.5% v/v, 1.0% v/v, and 5.0% v/v, respectively. The test was done in triplicates. The ideal fixation is chosen and applied in a toxicity test.
 |
Figure 2: Preparation of dilution series of copper sulfate at 7 concentrations; 5.0% v/v, 1.0% v/v, 0.5% v/v, 0.1% v/v, 0.05% v/v, 0.01% v/v, and 0.005% v/v. |
Preparation of Adsorbents and its Zeta Potential
Two waste materials, egg and shrimp shells were prepared. The eggshells represented Calcium Carbonate (CaCO3) and the shrimp shells represented Chitosan, which is the main component of each material. Both materials were tested for their surface charge. They were ground into a fine powder, diluted in distilled water, mixed with a vortex mixer, and the zeta potential was measured by Photon Correlation Spectroscopy (Delsa Nano C., Beckman Coulter). The experiment was done in triplicates.
Toxicity Test
Carbonyl phenol and copper sulfate at 1% v/v were prepared in flasks. Adsorbents (60g/L) were added to each flask equally. Samples were then taken out from the flask at 10 time points (30 minutes, 1, 2, 3, 4, 5, 6, 12, 24, 48 hours) and the toxicity test was conducted by bioassay. Escherichia coli (E.coli) were utilized as a bio-assay in the toxicity test. E.coli was developed to mid-exponential expression in LB broth. For each tube (2 samples at 10 time points), cultures were suspended in 1% v/v for 15 minutes and quantify the toxicity impacts by Miles and Misra technique [14]. For Miles and Misra technique, sample that contained E.coli after 15minutes were resusitate on LB agar plate and incubated at 35oC for 18 hours. The colonies were tallied and colony forming units (CFU) were recorded. Tests were done for 10 time points amd all tests were done in triplicates.
Table 1: Experimental set up for adsorption and toxicity testing
|
Flask |
Test Sample |
Adsorbent |
|
1 |
Carbonyl Phenol |
Egg Shell |
|
2 |
Carbonyl Phenol |
Shrimp Shell |
|
3 |
Copper Sulfate |
Egg Shell |
|
4 |
Copper Sulfate |
Shrimp Shell |
Results and Discussion
Optimum Sample Concentration
From the screening test, it was found that at 0.5% v/v there was some growth of bacterial cells, but there was 0% growth or a lethal effect at 1% v/v of both samples, copper sulfate and carbonyl phenol (Figure 3). Therefore, the suitable optimum concentration to conduct toxicity immobilization or an adsorption test was at 1% v/v as shown in Figure 4.
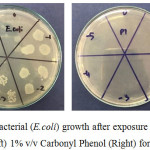 |
Figure 3: Bacterial (E.coli) growth after exposure to water control (Left) 1% v/v Carbonyl Phenol (Right) for 15 minutes. |
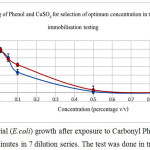 |
Figure 4: Bacterial (E.coli) growth after exposure to Carbonyl Phenol and Copper Sulfate for 15 minutes in 7 dilution series. The test was done in triplicates. |
Zeta Potential
The two types of adsorbents were tested for their surface charge and it was found that eggshells have a negative charge on the surface and shrimp shells have a positive charge on the surface as shown in Table 1.
Table 2: Zeta potential of tested adsorbents (egg and shimp shell)
|
Adsorbent Type |
Zeta Potential (mV) |
|
Egg shell |
-21.85 |
|
Shrimp shell |
+29.05 |
Toxicity Test
From the experiment, it was found that both adsorbents allowed bacteria (E.coli) to recover on the agar when plated out on LB following 15 minutes of exposure introduction, which showed the capacity to immobilized toxicity. The best duration for adsorption was found to be 12 hours. The eggshells as an adsorbent proved to be more effective in immobilizing toxicity compared to the shrimp shells in general. Bacteria culture recovered more than 60% and 50% on Copper Sulfate and Carbonyl Phenol, respectively. For the shrimp shells, the recovery rate was slightly lower at 34% and 9% for Carbonyl Phenol and Copper Sulfate, respectively (Figure 5).
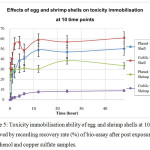 |
Figure 5: Toxicity immobilisation ability of egg and shrimp shells at 10 time observed by recording recovery rate (%) of bio-assay after post exposure to the phenol and copper sulfate samples. |
The experiment was done in triplicates.
Conclusion
Adsorption pre-treatment process employing waste materials such as egg and shrimp shells research was carried out. The optimum condition was when adsorbents were exposed to the toxic chemicals, copper sulfate and carbonyl phenol for 12 hours. Subsequently, an acute toxicity test was carried out by exposing E.coli to the treated toxic chemicals for 15 minutes and recovered on the agar. From the results, it was found that both chemicals were lethally toxic to E.coli. However, egg and shrimp shells proved the ability to immobilize toxicity and allow biological treatment. In general, eggshells were more effective in immobilizing toxicity. To be precise, eggshells, which are negatively charged on the surface from zeta potential experiment, enabled it to immobilize 62% toxicity of carbonyl phenol, which is positively charged, better than copper sulfate at 50%, which is negatively charged. Similarly, shrimp shells, which are positively charged on the surface, immobilize copper sulfate by 34%, which is negatively charged, better than carbonyl phenol by 9% (Figure 5). This shows some connection between surface charges to the ionic charge of the sample. This information could be useful in selecting a suitable adsorbent for the different types of wastewater and the buffering of toxicity could be a very effective way of aiding biological treatment. Future work will focus on more details of adsorbent characteristic identification and its mechanism. Then, these waste natural materials could potentially be used to manage the toxicity of real world waste that is toxic to microbial cells.
Acknowledgment
This work was partially supported by Faculty of Science and Technology, Rajamangala University of Technology Thanyaburi.
References
- United States Census Bureau (2013). Total Midyear Population for the World: 1950-2050. United States Census Bureau [online] Available at: <http://www.census.gov/population/international/data/idb/worldpoptotal.php> [Accessed: 20th November 2017].
- Malaeb, L; Ayoub, G. M. “Reverse osmosis technology for water treatment: State of the art review”. Desalination. 2011, 267, 1-8
CrossRef - Gutiérrez, G.; Lobo, A.; Benito, J. M.; Coca, J.; Pazos, C. “Treatment of a waste oil-in-water emulsion from a copper-rolling process by ultrafiltration and vacuum evaporation”. Journal of Hazardous Materials. 2011, 185, 1569-1574
CrossRef - Chipasa, K. “Best Practice Guide for the Disposal of Water-Mix Metalworking fluids”. United Kingdom Lubricants Association (UKLA). 2011, [online] Available at: <http://www.ukla.org.uk/documents/Best%20Practice%20Guide_Final.pdf> [Accessed: 6th November 2017].
- Byers, J. P. “The Chemistry of Metalworking Fluids. In: Metalworking Fluids”. United States of America: CRC Press. 2006, 128-141
- U.S. Environmental Protection Agency (EPA), “Green Chemistry”, EPA, 2012, [online] Available at: <http://www.epa.gov/greenchemistry/> [Accessed: 16th November 2017].
- Gu, B.; Schmitt, J.; Chen, Z. ; Liang, L.; McCarthy, J. F. “Adsorption and Deporption of Natural Organic Matter on Iron Oxide Mechanisms and Models.” Environmental Science & Technology. 1994, 28, 38-46
CrossRef - Hui, K. S.; Chao, C. Y. H. ; Kot, S. C. “Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash.” Journal of Hazardous Materials. 2005, 1-3, 89-101
CrossRef - Iyoda, F.; Hayashi, S.; Arakawa, S.; John, B.; Okamoto, M.; Hayashi, H.; Yuan, G. “Synthesis and adsorption characteristics of hollow spherical allophane nano-particles”, Applied Clay Science. 2012, 56, 77-83.
CrossRef - Wang, S.; Peng, Y. “Natural zeolites as effective adsorbents in water and wastewater treatment”, Chemical Engineering Journal. 2010, 156, 11-24
CrossRef - Asmel, N. K.; Yusoff, A. R. M.; Krishna, S.; Lakkaboyana; Majid, Z. A.; Salmiati, S. “High concentration arsenic removal from aqueous solution using nano-iron ion enrich material (NIIEM) super adsorbent”, Chemical Engineering Journal, 2017, 317, 343-355
CrossRef - Uapipatanakul, B. “Harmonising metal working fluid formulations with end-of-life biological treatment”. University of Oxford PhD Dissertation. 2015, No. 10087782
- El–Moselhy, K. M.; Abdel-Azzem, M.; Amer, A.; Al–Prol1, A. E. “Adsorption of Cu(II) and Cd(II) from Aqueous Solution by Using Rice Husk Adsorbent”. Physical Chemical: An Indian Journal, 2017, 12, 109
- Miles, A. A.; Misra, S. S.; Irwin, J. O. “The estimation of the bactericidal power of the blood.” Journal Of Hygiene, 1938, 38(6), 732-749.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










