Synthesis, Characterization of Copper Metavanadate (CuV2O6) Nanostructures via Hydrothermal Method and the Photocatalytic Performance
S. Rajendra Prasad1,2, S. Srikantaswamy2, K. Jagadish1,2, M. R. Abhilash2 and M. B. Nayan2
1Department of studies in Chemistry, Manasagangothri, University of Mysore-570006, Karnataka, India.
2Department of studies in Environmental Science, Manasagangothri, University of Mysore-570006, Karnataka, India.
Corresponding Author E-mail: srikantas@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/340309
Article Received on : December 21, 2017
Article Accepted on : March 03, 2018
Article Published : 06 May 2018
The nanostructures with different morphology have enormous applications in science and technology field, due to its vast advantages over normal materials. Here in, the research work has been carried out for the synthesis of novel nanostructures via hydrothermal method. The selection of nanomaterials was started with focus on the transition metal vanadates with high photocatalytic activity. The present work was carried out for the synthesis of copper metavanadates (CuV2O6) with different morphology like spherical and leaf like nanoparticle by hydrothermal method. The crystalinity and morphological properties were analyzed by using advanced instruments XRD and SEM respectively. Further, the photocatalytic properties of synthesized nanoparticle was investigated by made treatment with synthetic wastewater which contains the methyl red dye and heavymetals in it. The treatment was carried out for the different concentration of catalyst, methyl red dye, and heavymetals. However the effect of concentration of catalyst, pH, Dye concentration and heavymetal concentration was also investigated and founded the optimum concentration for the dye degradation and heavy metal removal simultaneously. This copper metavanadate with different morphology has high photocatalytic performance and the results were obtained with high efficiency.
KEYWORDS:Copper metavanadates; Hydrothermal; Photocatalyst; Wastewater
Download this article as:| Copy the following to cite this article: Prasad S. R, Srikantaswamy S, Jagadish K, Abhilash M. R, Nayan M. B. Synthesis, Characterization of Copper Metavanadate (CuV2O6) Nanostructures via Hydrothermal Method and the Photocatalytic Performance. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Prasad S. R, Srikantaswamy S, Jagadish K, Abhilash M. R, Nayan M. B. Synthesis, Characterization of Copper Metavanadate (CuV2O6) Nanostructures via Hydrothermal Method and the Photocatalytic Performance. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=45540 |
Introduction
The nanomaterials are having wide range of applications in different science fields such as photocatalysis, catalysis, energy, electronics, medicines etc. Transition metal particles with vanadate compound form different crystal structure with fascinating morphology, high photocatalytic performance. The transitional metal vanadates with nanostructure have different functions such as electronic, coloring agent, semiconductor, magnetic and medicinal etc [1-2]. Further the transition metal vanadates are used in catalysis, batteries and implantable cardiac defibrillators (ICDs) [2-4]. Dyes of different structure with organic properties including methyl red dye use in many industries pollute the whole aqua system in earth [5]. The different dyes were used in different textile industries introduce various colour dye to water which can create environmental pollution [6-10]. Among different dyes, the uses of methyl red dye is used in different textile industries. However the heavy metal is the hazardous element present in the industrial wastewater and also in other drinking water resources such as in ground water. Therefore removal of heavy metal and degradation of dyes present in wastewater is a challenging task for environmental chemist [11, 12].
The different treatment methods were, being adopted for dye degradation and heavy metal removal present wastewater such as adsorptive degradation by biological method and UV/Vis light photocatalysis [13-16]. The complete methyl red dye degradation carried out by photodegradation process, in which the vanadates will adsorb the dye molecules and helps for degradation in the presence of sunlight. Massive research work has been paying awareness towards the purification of industrial wastewater which contains the impurities such as organic dyes, inorganic heavy metals and etc. Simultaneous removal of these heavy metal and organic dyes is the challenging. Nowadays the research work focusing towards the simultaneous removal of dyes and heavy metals using transition metal-vanadate nanoparticles. However, the presence of heavymetals like Pb, Cr, Zn and Fe in industrial wastewater, still at small concentrations and their access into the atmosphere can be poisonous to living systems. The acceptance boundary of the metal ions in wastewater is 3.0mg L-1 [17]. The pollutants like dyes and heavymetals found together in wastewater, it suggests that the researchers should concentrate on the removal of these pollutants together [18-21]. The mainly used techniques for the removal of heavy metals from effluents consist of precipitation, evaporation, ion exchange, membrane filtration and adsorption. Among all the different methods adsorption is selected because of its cost effectiveness towards the removal of heavy metals [22-28].
Numerous techniques/methods have been invented for the synthesis of copper metavanadate nanostructures, such as hydrothermal, co-precipitation and solution method. [29-30]. Amongst, the hydrothermal method has much applicable because of its low cost, small reaction time duration with high-quality crystalline products. From the literature survey, it is explained that numbers of reports available about the production and photocatalytic performance of nanometal vanadates. Conversely, to the best of our understanding, there is no report published so far concerning the synthesis of copper metavanadate nanoparticles by hydrothermal method and their photocatalytic properties in wastewater treatment. Here in, the research work has been carried out by most suitable hydrothermal method to synthesis CuV2O6 nanoparticles, with their visible light photocatalytic performance. The advanced analytical techniques such as SEM with EDX, XRD, UV–visible, and COD analyzer have been utilized to analyze the nanoparticles. The prepared nanoparticles have been used to treat the synthetic wastewater which contains the heavy metals and methyl red (Figure 1) dye. Further the investigation of effect of pH, effect of Copper vanadates concentrations, and efficiency of photocatalysis were also performed.
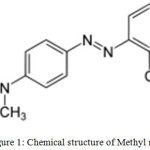 |
Figure 1: Chemical structure of Methyl red |
Materials and Methods
The commercially available water soluble dyes methyl red (λmax 410nm) were obtained from Sisco Research Laboratory Pvt. Ltd. The chemicals like copper nitrate trihydrate (Cu(NO3)2 3H2O) (99% A. R.), and Sodium metavanadate (NaVO3) are obtained from Sisco Research Laboratory Pvt. Ltd. The visible spectrophotometer (Shimadzu UV-1800) has been used for recording absorbance at λmax. Without further purification, above said all the reagents and solvents were used for the experiment. The typical experiment was proceeded by using 20 mL capacity Teflon-lined stainless steel Moray type autoclaves which was performed under autogenous pressure.
Synthesis of Copper Metavanadates
The CuV2O6 (Copper metavanadate) nanoparticles were synthesized by hydrothermal method. In a typical synthesis, Sodium metavanadate (0.258 g, 0.1 mol), Copper nitrate (0.48 g, 0.1 mol) and 20 mL of deionized water were mixed and mixed reactants were stirred at room temperature for 120 min before hydrothermal reaction. The stirred solution was transferred to clean, dried Teflon liner with maximum 20 ml capacity. The Teflon liner with ingredients was fixed in stainless steel moray type autoclaves and the autoclaves were placed in hot air oven for the duration of 5 hours at the constant temperature 160°C. Then after, the autoclaves were cooled down to room temperature slowly and Teflon liners were opened to get the dark brown color product of CuV2O6. Further the product was washed with distilled water and centrifuged to remove any soluble impurities; again the product was washed with small amount of ethyl alcohol, centrifuged and dried at 60°C in hot air oven. Finally the brown coloured solid was isolated, calcined at 400°C and characterized using different advanced analytical methods. The calcined material was used for the further treatment processes.
Characterization Techniques
The obtained brown coloured solid sample was analyzed using following analytical techniques. Scanning Electron Microscope characterization was carried out by ZEISS SEM microscope, model-evo/IS 15, made in Japan. The micro images were picked up at 5 kV and prior the material was coated with graphite for the well result. The absorbance of material was characterized by using instrument UV-Vis Shimadzu UV-1800, with cell volume-3 mL and cell length-10 mm. X-ray diffraction of synthesized material was carried out by the X-ray powder diffractometer with Cu Kα radiation, (λ=1.5406 Å) as the energy source. The characterization of heavy metal concentration was carried out by atomic absorption spectroscopy (model number GBC, Avanta version 1.31). A chemical oxygen demand (COD) analysis was carried out for the estimation of organic percentage present in the synthetic wastewater which indicates the dye presence, before and after photocatalytic treatment.
The Experimental Procedure For The Treatment Of Synthetic Wastewater
The obtained CuV2O6 nanoparticle was treated with different synthetic dye and heavymetal polluted water for the investigation of photocatalytic properties. The photocatalytic experiments were carried out under direct sunlight. The synthetic wastewater with different known concentration of dye and heavy metals solutions were prepared by dissolving methyl red and Pb, Fe, Cr and Mn heavy metals in 100ml water. The synthesized nanoparticle with different concentration was added to the synthetic wastewater, the mixture was irradiated under sunlight and simultaneously the shaking of mixture was carried out using mechanical shaker to increase the reaction of nanoparticles and polluted water interaction. The treatment was used to investigate the decolourization of polluted water in the presence of copper vanadates nanoparticle at different photocatalyst dosages and pH levels. Initially, 100ml of 100ppm of methyl red dye and 100 ppm of Pb, Fe, Cr and Mn heavy metals were tested with different catalyst dosage (from 0.1g to 1g). The optimum concentration of catalyst was investigated and using this optimum concentration of catalyst the pH was varied (from pH 2 to pH 11). The optimum pH level was investigated and by maintaining the constant pH level the dye concentration (10 ppm to 100 ppm) and heavymetal concentration (10 to 100 ppm) were varied to investigate the pahotocatalytic properties of nanoparticle. The pH of the effluent solution was varied by the adding 0.1 molar HCl and 0.1 molar NaOH solutions. Except UV and dark conditions all the experiments were carried out in the presence of direct sunlight. The whole experimental set-up was placed in sunlight between 11 a.m. and 5 p.m for the duration of 6 hours. The decolourization of dyes after degradation and before degradation was recorded using UV-Vis spectrophotometer to know the optimum catalyst concentration. Finally the removal of heavy metals were found by filtering the treated solution and analyzed by using AAS, also dye degradation properties were investigated using Chemical oxygen demand (COD).
Results and Discussion
X-ray Diffraction (XRD)
Figure 1 shows the typical XRD pattern of the as-prepared CuV2O6 nanoparticles. All the diffraction peaks can be readily indexed to the JCPDS No. 73-1032. The obtained diffraction peaks 24.7o, 26.4o, 28.2o, 28.9o, 29.2o, 33.9o, 35.9o, 38.1o, 39.1o, 42.8o, 43.3o, 46.58o, 49.4o, 52.4o, 54.2o, 57.2o, 58.9o, 61.4o, 65.1o and 68.24o, corresponding to (110), (112), (020), (021), (200), (112), (113), (022), (222), (131), (004), (313), (132), (133), (225), (423), (316), (335) and (243) planes respectively (Figure 2). The obtained product does not show any other peak except the CuV2O6 nanoparticles indicating that the less impurities.
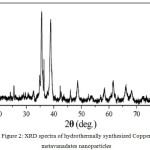 |
Figure 2: XRD spectra of hydrothermally synthesized Copper metavanadates nanoparticles
|
Morphology
The morphology of the synthesized CuV2O6 nanoparticles was characterized by Scanning electron microscopy (SEM), which is shown in Figure 3. The SEM image was taken in different magnification for the identification of surface morphology of copper metavanadates. It was observed that, hydrothermally synthesized nanoparticles have shown the different morphology with spherical shape with nanoleaf like structure. In hydrothermal condition the ingredients are mixed and reacted under pressure which can convert from agglomeration to nanosize and plane surface to porous like materials. The distribution of copper metavanadate nanoparticles in the solution was high with nanosize of 50 nm and this nanosize with high surface area is responsible for the high photocatalytic activity which was proved by treatment method of synthetic wastewater.
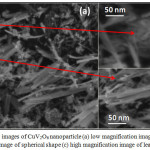 |
Figure 3: SEM images of CuV2O6 nanoparticle (a) low magnification image (b) high magnification image of spherical shape (c) high magnification image of leaf like shape Click here to View figure |
Absorbance Spectrum
UV-Vis analysis suggests the successful adsorption of the dye on the surface of the copper metavanadates nanoparticle. The absorbance values in the visible region were taken for methyl red dye which was used in synthetic wastewater, as shown in Figure 3. The methyl red solution was prepared with suitable solvent and the supernatant solution was used to carry out the UV-Vis analyses (Figure 4). The absorbance procedure was carried out for methyl red dye in the wavelength range of 200 to 800 nm and the solution shown the absorbance maximum peak at nearly 410 nm. The absorption spectra indicates that the degradation efficiency with the wavelength region of 200 to 800 nm.
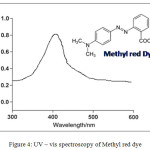 |
Figure 4: UV – vis spectroscopy of Methyl red dye
|
Effect of catalyst concentration:
Catalyst concentration studies for the degradation of methyl red dye was investigated by following method. The percentage of dye degradation was estimated by analyzing the UV-vis absorbance of synthetic wastewater before and after treatment, similarly the percentage of heavymetals was estimated by Atomic absorption spectroscopy analysis. The amount of catalyst CuV2O6 nanoparticle varies from 0.1g to 1g/100ml (Table 1). The percentage of degradation of dye and heavy metal removal has shown appreciable results. Where, copper vanadates photocatalyst showed maximum of 98.70 % degradation and 96.10 % adsorptive removal at 0.5g/100ml (Figure 5). The copper vanadates with the nanoparticle size of 50nm is sufficient to degrade the dyes by photocatalytic reaction and remove heavy metals by adsorption process. By increasing the amount of catalyst the nanoparticles agglomeration will form and the availability of photocatalyst surface area become low and shows less photocatalytic activity for dye degradation and less adsorption for heavy metal removal. Thus we can say, the degradation is also depending on the size of the nanoparticles and the optimum catalyst concentration was found that 0.5 g.
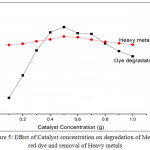 |
Figure 5: Effect of Catalyst concentration on degradation of Methyl red dye and removal of Heavy metals Click here to View figure |
Table 1: Effect of catalyst Copper metavanadate concentration on the degradation of methyl red dye and Heavy metals removal
|
Catalyst concentration (g) |
Percentage (%) of dye degradation |
Percentage (%) of heavy metal removal |
|
0.1 |
79.40 |
93.78 |
|
0.2 |
85.50 |
94.12 |
|
0.3 |
92.30 |
94.78 |
|
0.4 |
97.20 |
95.30 |
|
0.5 |
98.70 |
96.10 |
|
0.6 |
97.00 |
95.88 |
|
0.7 |
96.80 |
95.24 |
|
0.8 |
94.20 |
94.64 |
|
0.9 |
92.10 |
94.32 |
|
1.0 |
90.70 |
93.88 |
Effect of pH at catalyst concentration 0.5g
Effect of pH on the degradation of methyl red dye was investigated by varying the pH from 2 to 11 (Table 2) using the optimum catalyst concentration of 0.5 g/100ml. The results illustrated that, the degradation efficiency affected by pH (Figure 6). The percentage of degradation on methyl red dye increased from 88.40% to 98.60% from pH 2 to pH 5 and decreased to 85.10% at pH 11. Effect of pH explained on the basis of charge of copper metavanadates nanoparticles, where charge of copper metavanadates is positive. Copper metavanadate nanoparticles surface is positively charged with below the pH 5. The methyl red is an anionic dye, the surface was attracted more towards the surface of catalyst and degradation is more.
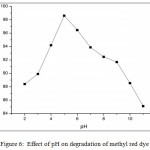 |
Figure 6: Effect of pH on degradation of methyl red dye Click here to View figure |
Table 2: Effect of pH on degradation of methyl red dye
|
pH |
Percentage (%) of dye degradation |
|
2 |
88.40 |
|
3 |
89.90 |
|
4 |
94.20 |
|
5 |
98.60 |
|
6 |
96.46 |
|
7 |
93.88 |
|
8 |
92.46 |
|
9 |
91.68 |
|
10 |
88.54 |
|
11 |
85.10 |
Effect of dye and heavy metal concentration at catalyst concentration 0.5g and pH 5
Further, Experiments were conducted to study the effect of methyl red dye and heavy metal concentration by varying the methyl red dye concentration from 10 to 100 ppm (Figure 7a) and heavy metals concentration from 10 to 100 ppm (Figure 7b). The results obtained for copper vanadates at 0.5g, at pH 5 and found 96.70, 98.30, 99.10, 96.54, 94.30, 92.14, 91.46 90.84, 90.50 and 90.38 % for dye degradation (Table 3). When the dye concentration increases, the amount of dye adsorbtion increased, radiation photons from sunlight to the catalytic surface decreases and the photons get intercepted before they can reach the catalyst surface, decreasing the absorption of photons by the catalyst and the equilibrium adsorption of dye on the catalyst surface which results in a decrease in the active sites. These activities results in the decreased photocatalytic reaction rate as there will be lower photon adsorption for the catalyst particles. When the Heavy metal concentration increases, the adsorption of heavymetals also increases for upto the 80 ppm (Table 4), further the treated solution was filtered to remove heavy metals from the synthetic wastewater. After filtration the solution was analyzed using AAS and found that removal was low at above 80ppm, this may due to the increasing the positive charge of heavy metals in the surrounding of catalyst and get repulsion each other.
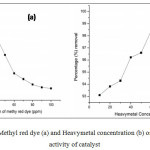 |
Figure 7 : Effect of Methyl red dye (a) and Heavymetal concentration (b) on the photocatalytic activity of catalyst Click here to View figure |
Table 3: Effect of Methyl red dye concentration on the photocatalytic activity of catalyst
|
Dye concentration (ppm) |
Percentage (%) of degradation |
|
10 |
96.70 |
|
20 |
98.30 |
|
30 |
99.10 |
|
40 |
96.54 |
|
50 |
94.30 |
|
60 |
92.14 |
|
70 |
91.46 |
|
80 |
90.84 |
|
90 |
90.50 |
|
100 |
90.38 |
Table 4: Effect of Heavy metal concentration on the photocatalytic activity of catalyst
|
Heavy metals concentration (ppm) |
Percentage (%) of removal |
|
10 |
93.10 |
|
20 |
93.82 |
|
30 |
94.28 |
|
40 |
96.22 |
|
50 |
96.60 |
|
60 |
98.48 |
|
70 |
99.10 |
|
80 |
99.60 |
|
90 |
96.88 |
|
100 |
96.14 |
Summary and conclusion
In the present research work, the effort has been made to investigate the photocatalytic performance and adsorption capacity of copper metavanadate nanoparticles (CuV2O6). The CuV2O6 nanoparticles were synthesized by hydrothermal method, which was cost effective and helpful in the tuning of morphology and size of the product by applying autogenous pressure on the ingredients in hydrothermal condition. However hydrothermally tuned CuV2O6 nanoparticles shows good crystalline properties with leaf like and spherical like structure. Leaf like morphology helpful in the adsorption of heavymetal and spherical like morphology helpful in the adsorption as well as high photocatalytic activity towards the degradation of methyl red dye. Both the adsorptive degradation of dye and adsorptive removal of heavymetal shows high efficiency. This type of novel nanomaterials is needed for the present research trends and also the author focused on the synthesis of this type of novel transition vanadates with specific morphology.
Acknowledgements
The author’s are carried out the research work with financial support of University with potential for excellence and Center with potential of excellence in particular area, major research project granted by University Grant Commision to University of Mysore, Mysuru.
Reference
- Masato, M.; Yui, M.; Yuichi M.; Keita I.; Chem. Commun. 2011, 47, 9591.
CrossRef - Valeria, C.; Floris, B.; Dalton Trans. 2011, 40, 1419.
CrossRef - Mai, L. Q.; Xu, L.; Han, C.H.; Xu, X.; Luo, Y. Z.; Zhao, S, Y,; Zhao, Y. L.; Nano Lett. 2010, 10, 4750.
CrossRef - Crespi, A. M.; S.K. Somdahl, C.L. Schmidt, P.M. Skarstad, J.Power Sources, 2001, 96, 33.
CrossRef - Grau, P.; Water Sci. Technol., 1991, 24(1), 97–103.
CrossRef - Rieger,P. G.; Meir, H. M.; Gerle, M.; Vogt, U.; Groth, T.; and Knackmuss, J. H.; Biotechnol., 2002, 94, 101.
- Liu, G. M.; Li, X. Z.; and Zhao, J. C.; Environ. Sci. Technol., 2000, 34, 3982.
CrossRef - Habibi, M. H.; Hassanzadeh, A.; and Mahdavi, S.; J. Photochem. Photobiol., A, 2005, 172, 89.
CrossRef - Hu, C.; Hu, X. X.; Wang, L. S.: Qu, J. H.; and Wang, A. M.; Environ. Sci. Technol., 2006, 40, 7903.
CrossRef - Janus, M.; and Morawski, A. W.; Appl. Catal., B, 2007, 75, 118.
CrossRef - Jagadish, K; Chandrashekar, B. N.; Byrappa, K.; Rangappa, K. S.; and Srikantaswamy, S.; Anal. Methods, 2016, 8, 2408–2412.
CrossRef - Zhang, H.; Chen, D.; Xiaojun, L. V.; Wang, Y.; Chang. H.; and J. Li, Environ. Sci. Technol., 2010, 44, 1107.
CrossRef - Sun, X. J.; Wang, J. W.; Xing, Y.; Zhao, Y.; Liu, X. C.; Liu, B.; Hou, S. Y.; Cryst. Eng. Commun. 2011, 13, 367.
CrossRef - Pengfei, J.; Zhang, J.; Chen F.;and Anpo, M.; Appl. Catal., B, 2009, 85, 148.
CrossRef - Cervantes, F. J.; Espinosa, A. G.; Moreno-Reynosa, M, A,; and Rangel-Mendez, J. R.; Environ. Sci. Technol., 2010, 44, 1747.
CrossRef - Fernandez, J.; Bandara, J.; Lopez, A.; Buffat, P.; and Kiwi, J.; Langmuir, 1999, 15, 185–192.
CrossRef - Lee, J.; Shim, H. S.; Lee, M.; Song, J. K.; and Lee, D.; J. Phys. Chem. Lett., 2011, 2, 2840.
CrossRef - Zhang, Y.; Bai, Y.; and Yan, B.; Drug Discovery Today, 2010, 15, 428–435.
CrossRef - Awwad, A. M.; and Farhan, A. M.; Am. J. Chem., 2012, 2, 238–244.
CrossRef - Aytar, P.; Gedikli, S.; Buruk, Y.; Cabuk. A.; and Burnak, N.; Int. J. Environ. Sci. Technol., 2014, 11(6), 1631–1640.
CrossRef - Baig, K. S.; Doan, H. D.; and Wu, J.; Desalination, 2009, 249, 429–439.
CrossRef - Mohan, D.; Pittman Jr. C. U.; and Steele, P. H.; J. Colloid, Interface Sci., 2006, 297, 489–504.
CrossRef - Papadopoulos. A.; Fatta, D.; Parperis, k.; Mentzis, A.; Haralambous, K. J.; and Loizidou, M.; Sep. Purif. Technol., 2004, 39, 181–188.
CrossRef - Ku, Y.; and Jung, I. L.; Water Res., 2001, 35, 135–142.
CrossRef - Chan, B. K. C.; and Dudeney, A. W. L.; Miner. Eng., 2008, 21, 272–278.
CrossRef - Fu, F.; and Wang, Q.; J. Environ. Manage., 2011, 92, 407–418.
CrossRef - Murthy, Z. V. P.; and Chaudhari, L. B.;Chem. Eng. J., 2009, 150, 181–187.
CrossRef - Kuo, C. Y.; and Lin, H. Y.; Desalination, 2009, 249, 792–796.
CrossRef - Swayampakula, K. M.; Boddu, V.; Nadavala S. K.; and Abburia, K.; J. Hazard. Mater., 2009, 170, 680–689.
CrossRef - Zhang, S.Y.; Ci, L.J.; Liu, H.R.; J. Phys. Chem. C, 2009, 113, 8624.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









