Synthesis and Characterization of Ni-Cu Doped Zirconia-pillared Bentonite
Ahmad Suseno1 , Karna Wijaya2, Wega Trisunaryanti2 and Roto2
, Karna Wijaya2, Wega Trisunaryanti2 and Roto2
1Department of Chemistry, Faculty of Sciences and Mathematics, Diponegoro University, Jl. Prof Soedarto, SH Tembalang, Semarang 50275, Indonesia.
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, Gadjah Mada University,Sekip Utara BLS 21, Bulaksumur, Jogjakarta, Indonesia.
Corresponding Author E-mail: karnawijaya@ugm.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340332
Article Received on : November 30, 2017
Article Accepted on : February 13, 2018
Article Published : 04 May 2018
Cu doped Ni/zirconia-pillared bentonite prepared using pillarization-impregnation method and its application as heterogeneous catalyst for hydrocracking of cooking oil to biogasoline has been investigated. Several zirconia-pillared bentonite supported Ni-Cu catalyst (Ni-Cu [x:y])/ zirconia-pillared bentonites; where x and y represent the mass ratio of Ni and Cu, respectively) were prepared by the impregnation method and used for hydrocracking of cooking oil to biogasoline. The Ni-Cu/zirconia-pillared bentonite samples were characterized by TEM, SEM, TGA, while the surface area by N2 adsorption, GC-MS was used to evaluate the product. The catalytic activity was assessed given the effects of different mass ratios of Ni and Cu. Among all the samples, the Ni-Cu(3:1)/zirconia-pillared bentonite catalyst showed the highest catalytic ability.
KEYWORDS:Zirconia-Pillared Bentonite; Ni-Cu Catalysts; Mass Ratios; Biogasoline
Download this article as:| Copy the following to cite this article: Suseno A, Wijaya K, Trisunaryanti W, Roto R. Synthesis and Characterization of Ni-Cu Doped Zirconia-pillared Bentonite. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Suseno A, Wijaya K, Trisunaryanti W, Roto R. Synthesis and Characterization of Ni-Cu Doped Zirconia-pillared Bentonite. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=45365 |
Introduction
The supports are used for deposition of the nickel phase range from conventional carriers, i.e., TiO2, SiO2, Al2O3 and ZrO2, to become mixed oxide systems and molecular sieves.1,2 We have shown3 that nickel can also be deposited within the highly porous structures of pillared interlayered clays (PILCs). PILCs are synthesized from natural layered minerals, such as bentonite, by replacement of the interlayer cations with positively charged oxohydroxy oligomeric species of the desired pillar-forming cation.4 After calcination, the cationic oligomers turn into oxidic pillars firmly bound to the silicate sheets leading to rigid porous structures opened to gases and vapours. Alumina-, Titania- and Zirconia-pillared clays are among the most widely studied.4 In this study, we report the use of bimetallic Ni-Cu catalysts for the catalytic hydrocracking of cooking oil of palm. Ni is a cheap metal compared to noble metals and is known for its high hydrogenation activity for a broad range of organic groups. Cu is applied as a promoter, primarily to improve the distribution of Ni on the support, and to prevent excessive carbon deposition on Ni.5 Nickel and copper can be incorporated into the PILC structure during the pillaring procedure. This work aimed to design and synthesized supported Nickel and Copper catalysts using zirconia-pillared bentonite as a carrier. The obtained materials were tested hydrocracking of palm cooking oil.The catalytic results were being discussed regarding the physicochemical properties of the samples.
Experimental
A sample of natural clay was collected from an open clay deposit in Boyolali, Central Java, Indonesia.The commercial zirconia chloride ZrOCl2•8H2O of industrial grade was supplied by Jiaozou Huasu Chemical Co.Ltd (purity 99%). The analytical grade of Ni(NO3)2•6H2O and Cu(NO3)2• 3H2O were obtained from Merck.All chemicals werw used without further treatment. Starting from ZrO2-pillared bentonite was prepared by following the procedure described4,6. The transformation of natural bentonite into pillared clay required the use of pillaring solution prepared by 0.10M ZrOCl2•8H2O. The pillared clay was air-dried at ambient temperature and 80°C for 8 hours, then calcinated at 400°C for 4 hours. The product is referred to as BZR. Ni-Cu samples were prepared by the wet impregnation method,where Cu(NO3)2•3H2O and Ni(NO3)2•6H2O were mixed to obtain the following Ni:Cu molar ratios: 1:0, 3:1, 1:1, and 1:3.Every solution should contain as much as metal nitrate to get 2 wt% metal in the final catalyst powder. The synthesis catalyst had the same composition as sample of NiCu11/ZrO2-pillared bentonite and was designated as Ni-Cu11/BZR in the following discussion. Finally, the pellet catalyst was obtained by a pellet printing device. Catalytic activity tests were performed in a continuous tubular fixed-bed micro-reactor with a catalyst weight, temperature, and H2 gas rate of of 0.1 g, 350oC, and 20 cc/s, respectively. The Characterization of the specific surface area and porosity of the bentonite used N2 adsorpsi-desorpsi method (BET NOVA 1994-2010 Instrument version 11:0). The morphology and elemental analysis of the test samples were estimated by SEM-EDX (JEOL JED-2300 Analysis Station, 20 kV) and TEM (JEOL JSM -6510 at 100 kV). The product was analysed by GC-MS (GCMS-QP2010 plus) to confirm the biogasoline formation in this reaction.The thermal analysis method (DTA) was used to distinguish the type of coke deposit formed on the surface of the used spent catalyst.
Results and Discussion
Texture Properties
The adsorption isotherms give a good fit on the BET equations for the Zr-pillared bentonite materials. Furthermore, Table 1 shows an increase in surface area along with an increase in the ratio of Cu content obtained from a plot of BET equation with different pore size distribution.The increasing ratio of Cu content (NiCu13) has led to different interactions so that the pore size distribution and the surface area become larger. In contrast, the increased Ni content ratio (NiCu31/BZR) results in a lower surface area than before the addition of Cu (Ni/BZR)7,8,9. This situation was due to pore blocking by metal species dispersed in the interlayer of supports material10,11.The composition of Ni-Cu ratio becomes strategic because it affects the catalyst activity as shown in the fig.4, table.1.
Table 1: Textural properties of ZrO2-pillared Bentonite and Ni-Cu/BZR
| Sample | Zr (M) | Ni % | Ni-Cu % | SBET (m2/g) | Vp (cc/g) | Vµp (cc/g) | Pore Radius (Å) |
| BZR | 0.1 | – | – | 194.8 | 0.151 | 0.045 | 17.28 |
| Ni/BZR | 0.1 | 2.0 | – | 111.567 | 0.34 | 0.027 | 16.889 |
| NiCu31/BZR | 0.1 | – | 2.0(3:1) | 107.488 | 0.317 | 0.056 | 18.978 |
| NiCu11/BZR | 0.1 | – | 2.0(1:1) | 132.97 | 0.122 | 0.082 | 15.306 |
| NiCu13/BZR | 0.1M | – | 2.0(1:3) | 322.754 | 0.93 | 0.085 | 19.089 |
Microscopy Analysis
The results of the analysis using TEM show apparently that there is no damage to the pillared bentonite structure of ZrO26. The texture conditions between the layers of the bentonite still look right that the impregnation process of Ni and Cu metal does not cause bentonite destruction as support. Also, the used catalyst texture used has shows black blocking which is indicated as carbon compounds as in fig.1.
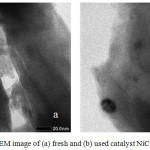 |
Figure 1: TEM image of (a) fresh and (b) used catalyst NiCu31/ BZR |
Strong indications of carbon deposits on used catalysts are strengthened in thermal analysis. DTA analysis suggests the energy release process (ΔH = negative) as well as weight reduction at temperatures around 400-600oC. The morphological analysis of the NiCu/ZrO2-Bentonite catalyst uses SEM method as shown in Fig.2. The figure shows the presence of layers on the surface of the used catalyst which reinforces the prediction that the carbon deposit is indeed present. The SEM image very clearly indicates a parallel correlation of TEM and DTA analysis which states that carbon deposits occur during the catalyst test process.
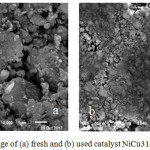 |
Figure 2: SEM image of (a) fresh and (b) used catalyst NiCu31/ BZR
|
Analysis of precursor of coke
The result of elemental analysis using EDX shows the previous assumption that there is an increase of carbon content (5.93% to 32.25%) in the catalyst after being used for hydrocracking reaction of palm cooking oil into biogasoline as shown in table 2. A more in-depth analysis of carbon deposits has been analyzed using DTA data showing in Fig.3 that coke releases from the catalysts that occur at different temperatures for each sample.
Table 2: Elemental analysis of Catalyst of NiCu31/ BZR
|
Elements |
NiCu31/BZR (fresh) % massa |
NiCu31X/BZR (used) % massa |
|
C |
5.93 |
32.25 |
|
Mg |
1.41 |
0.99 |
|
Al |
6.79 |
4.83 |
|
Si |
18.2 |
12.92 |
|
K |
0.39 |
0.38 |
|
Fe |
1.95 |
1.45 |
|
Ni |
2.86 |
2.03 |
|
Cu |
1.49 |
0.74 |
|
Zr |
16.67 |
11.14 |
The heat of coke combustion illustrates how high the interaction occurs on the cauldron surface with coke. The massive release of combustion energy symbolized by the combustion enthalpy is ΔH with joules/g12,13. Based on Fig.3 the temperature of the combustion process is divided into two parts. The first part occurs at a temperature interval of 350 – 400°C where ΔH for NiCu31/BZR and NiCu13 /BZR catalysts are -215.3553 and -76.2515 J/g, the coke at that temperature often referred to as softcoke. While the second part of the combustion process occurs at a temperature interval of 550-600°C, where ΔH for Ni/BZR and NiCu13/BZR catalysts are -1261.2932 J/g for the former and -1457.7797 J/g and the coke that burns at that temperature referred to as hardcoke.
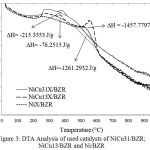 |
Figure 3: DTA Analysis of used catalysts of NiCu31/BZR; NiCu13/BZR and Ni/BZR |
The phenomenon of the coke combustion process on the catalyst surface may provide information on the catalytically activated model which for the NiCu31/BZR catalyst undergoes a slower deactivation process compared to NiCu13/BZR and Ni/BZR catalysts14.Thus the mass ratio of Ni:Cu metal catalyst of 3:1 are more resistant to deactivation caused by hydrocracking of palm cooking oil conversion reactions.
Catalytic cracking of palm cooking oil
The hydrocracking process using a catalyst aims to cut long, cyclic and branched saturated carbon chains to form carbon chains with a lighter molecular weight. Fig.4 shows the conversion of hydrocracking of cooking oil product at 350°C that includes gases, liquid hydrocarbon fuel and coke.
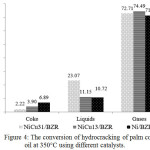 |
Figure 4: The conversion of hydrocracking of palm cooking oil at 350°C using different catalysts. Click here to View figure |
The liquid hydrocarbon fuel yield increases with increased mass ratio of nickel. The increase mass ratio of Ni to the NiCu/BZR catalyst could enhance the yield of hydrocarbon biofuel due to the increase of acid site, which will do transformation of fatty acid to hydrocarbon-based biofuels. The product of liquid hydrocarbons on NiCu/BZR catalysts has increased along with higher mass ratio Ni:Cu. However, Ni catalysts without the addition of Cu result in lower yields of the hydrocarbon product. These facts show that the synergic interactions between Ni and Cu to produce acid sites on the catalysts that promote cracking reactions of palm cooking oil during the conversion of triglyceride molecules into hydrocarbon-based biofuels15. The bimetallic catalysts are more active than the monometallic ones, an indication for a synergic effect between Ni and Cu. The coke increase that produced by the catalyst with a higher Cu content compared to a catalyst with a higher Ni content. This phenomenon may be due to the high Cu mass ratio of catalyst that causes high activity of producing aromatics.
Table 3: The product yield of biogasoline fraction
|
Catalyst |
Product Yield biogasoline % (w/w) |
| BZR |
8.64 |
| NiCu31/ BZR |
25.07 |
| NiCu13/BZR |
11.05 |
| Ni/ BZR |
8.72 |
The amount of the gasoline fraction yield of each liquid hydrocarbon fuel product is shown in Table.3 The amount of total biogasoline fraction is calculated based on the ratio of % (w/w) of the resulting liquid fraction, then done by fractionation distillation process at 130oC. Based on the GC-MS analysis, the distillation product is biogasoline fraction (C5-C12). Table.3 shows the catalyst selectivity to the highest gasoline fraction is indicated by the NiCu31/BZR catalyst. The metal ratio of 3:1 for Ni and Cu shows that the primary metal of the catalyst is Ni, where as Cu metal with a smaller amount of percentage is a promoter which can improve the catalytic function of Nickel metal. As a comparison for catalyst activity in producing biogasoline fraction on Ni/BZR catalyst produced only 8.72% which is of course much smaller than on NiCu31/BZR catalyst that of 25.07%.
Conclusions
The catalytic effect of Ni/BZR and Ni-Cu/BZR catalysts on textural properties and catalyst activity has shown an increase in surface area along with an increase in the Cu content ratio, however the ratio with high Ni content can increaseing the liquid hydrocarbon fuel product. Through TEM and SEM on the analysis on used catalysts indicates the presence of coke deposits that cover a portion of the zirconia pillared bentonite surface that causes deactivation process.The best outcome confirmsed the conversion of hydrocracking palm cooking oil and selectivity for biogasoline could be simultaneously more than 20% by employing the NiCu31/BZR catalyst at 350oC. Besides having excellent activity, the NiCu31/BZR catalyst also has a higher deactivation capability than other catalyst types.
Acknowledgements
This study was supported by Kemenristek Dikti, Republic of Indonesia. The financially support from a doctoral dissertation research scheme Grant No.345-14/UN7.5.1/PP/ 2017.
References
- Feng, Y.; Hao, J.; Liu, W.; Yao, Y.; Cheng,Y.; Xu H. Chin. Chem. Lett. 2015, 26, 709-713.
CrossRef - Liu, W.; Feng, Y.; Wang, G.; Jiang, W.; Xu, H. Chin. Chem. Lett. 2016, 27, 905-909.
CrossRef - Chen, M.; Fan, L.; Qi, L.; Luo, X.; Zhou, R.; Zheng, X. Catal. Commun. 2009, 10, 838–841.
CrossRef - Yamanaka, S.; Brindley, G.W. Clays Clay Miner. 1979, 27, 119-124.
CrossRef - Ambursa, M.M.; Ali, T.; Voon, L.H.; Lee H.; Sudarsaman, P .; Bhargava, S.K.; Abd Hamid, S.B. Fuel. 2016, 180, 767-776.
CrossRef - Suseno, A.; Wijaya, K.; Trisunaryanti,W.; Shidiq, M. Asian. J. Chem. 2015, 27, 2619-2623.
CrossRef - Fatimah, I.; Rubiyanto, D.; Kartika, N.C. Indones. J. Chem. 2016, 16 (1), 8-13.
- Gil, A.; Massinon, A.; Grange, P. Microporous Mater. 1995, 43, 69-75.
- Arfaoui-Rhouma, S.; Frini-Srasra, N.; Srasra, E. Afr. J. Pure Appl. Chem. 2011, 5, 339-345.
- Zhang, X.;Wang, T.; Ma, L.; Zhang, Q.;Yu, Y,; Liu, Q. Catal. Commun. 2013, 33, 15–19.
CrossRef - Botas,J.A.; Serrano, D.P.; García, A.; de Vicente, J.; Ramos, R. Catal. Today. 2012, 195, 59-70.
CrossRef - Urata, K.; Furukawa, S.; Komatsu, T. Appl. Cat. A: General. 2014, 475, 335–340.
CrossRef - Schmidt, F.; Hoffmann, C.; Giordanino, F.; Bordiga, S.; Simon, P.; Carrillo-Cabrera, W.; Kaskel, S. J. of Cat. 2013, 307, 238–245.
CrossRef - Kitla A.; Safonova, O.V.; Fottinger K. 2013, Catal. Lett. 2013, 143, 517–530.
CrossRef - Sangdara, P.; Subsadsana, M.; Ruangviriyachai, C. Orient. J. Chem. 2017, 33 (5), 2257-2262.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









