Synthesis and Antimicrobial Activity of Some new Pyrimidines of 6-Chlorobenzimidazoles
Indira M. Madawali , Navanath V. Kalyane, Gaviraj E. N and Shivakumar B
, Navanath V. Kalyane, Gaviraj E. N and Shivakumar B
Department of Pharmaceutical Chemistry, B.L.D.E.A’s SSM College of Pharmacy and Research Centre, Vijayapur, Karnataka,586103, India.
Corresponding Author E-mail: drbsk_2007@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340358
Article Received on : April 02, 2018
Article Accepted on : June 05, 2018
Article Published : 19 Jun 2018
A new series of pyrimidines of 6-chlorobenzimidazoles have been synthesized by the reaction of chalcone derivatives of 6-chlorobenzimidazole with guanidine nitrate in ethanol and aqueous solution of sodium hydroxide for evaluating them as potent antimicrobial agents. Results reveal that, compounds exhibited significant antibacterial and antifungal activities.
KEYWORDS:Antimicrobial Activity; Benzimidazoles; Chalcones; Pyrimidines
Download this article as:| Copy the following to cite this article: Madawali I. M, Kalyane N. V, Gaviraj E. N, Shivakumar B. Synthesis and Antimicrobial Activity of Some new Pyrimidines of 6-Chlorobenzimidazoles. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Madawali I. M, Kalyane N. V, Gaviraj E. N, Shivakumar B. Synthesis and Antimicrobial Activity of Some new Pyrimidines of 6-Chlorobenzimidazoles. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46707 |
Introduction
Benzimidazole nucleus is one of the bioactive heterocyclic compounds exhibiting a range of biological activities.1 Due to a wide range of pharmacological activities of benzimidazoles, synthesis has been found a significant attention now a day.
To, develop novel quinoline based fused heterocyclic systems; a quinoline nucleus with different substituent’s was required, which afforded a versatile synthetic procedure for further heterocycles.2
Synthesis of pyrimidines has been advanced and developing field in the heterocyclic chemistry. Pyrimidines have the crucial value of their biological and pharmacological properties. α, β-unsaturated ketones are reported to react with guanidine to give the unstable dihydropyrimidines which get dehydrogenated to form 2-aminopyrimidines.3
Pyrimidine ring complexes with various heterocyclic molecules and found to be an essential part of the natural products, agrochemicals and veterinary medical products. Fused pyrimidine derivatives have attracted many researchers over many years, due to their important biological activities.4 The preclinical data from the literature survey reveals that the heterocycles in conjunction with the pyrimidine have shown good antimicrobial,5 antioxidant,6 anti-inflammatory,7 analgesic and antipyretic,8 anti-tumor activities.9 In particular, bezimidazolo-pyrimidine derivatives were found as strong antimicrobial agents.10,11
Antimicrobial agents are one of the most important weapons in the resistance of infection caused by bacterial strains. In the last few years, increase the resistance of micro-organisms toward antimicrobial agents is a serious health problem, so there is a need for safe, effective and novel antimicrobial agents.12
By seeing impressive biological profile of benzimidazoles, quinolines and pyrimidines and also with respect to our work in synthesis and evaluation of biologically active new heterocycles, we planned to synthesize the new series of pyrimidines of 6-chlorobenzimidazoles as potential antimicrobial agents.
Materials and Methods
All the solvents and reagents were bought and used as such. The fusion points are examined by open capillary tube method and are uncorrected. By the use of KBr pellet technology IR spectra of compounds on SHIMADZU FTIR spectrometer 8400 S were noted. NMR spectra were seized on Bruker Avance II of 400 NMR spectrometer.
Synthesis of 6-chloro-2-(α-hydroxyethyl)benzimidazole (II)
An equimolar amount of 4-chloro-O-phenylenediamine (0.01 mole) and lactic acid (0.01 mole), 4N HCl are refluxed in synthetic microwave oven with the intensity of 65% (450 W) for a period of 190 minutes. The conclusion of the reaction was supervised by TLC, and then the reaction mixture was neutralized with sodium bicarbonate. The filtered product was washed and dried with water. Further, it was purified by recrystallization from ethanol. m.p. 194-95ċ.13,14,15
Synthesis of 6-chloro-2-acetylbenzimidazole (III)
To a reaction mixture of 6-chloro-2-(α-hydroxyethyl)benzimidazole (9.8g, 50 mmol) in Dilute H2SO4 (5% ; 40 ml) with stirring, a solution of K2Cr2O7 (44g, 150 mmol) in aqueous H2SO4 (25%, V/V; 80 ml) was taken up during a period of 20 minutes drop wise. Continuing the agitation for 2 hours at an ambient temperature, the solid (which is the chromium complex) separates out, was suspended in 50 ml of water. The pH up to 6-6.5 was adjusted with aqueous ammonia (1:1). Then the solid product filtered, washed with water and dried. The product further recrystallized from ethyl acetate. m.p. 198-99ċ.16
General procedure for the synthesis of chalcone derivative of 6-chlorobenzimidazoles (IVa)
To a reaction mixture of 6-chloro-2-acetylbenzimidazole (10mmol, 1.94g) in aqueous NaOH (10%, 30 ml), respective 2-chloroquinoline-3-carbaldehyde (10mmol, 1.91g) were added and agitated for 30 minutes at an ambient temperature. After the conclusion of the reaction the solid product was filtered, washed and dried with water. Further, it was recrystallized from ethanol.17, 18,19
Chalcone derivatives of 6-chlorobenzimidazoles (IVa-k)
IVa : Yellow solid, yield 76%, mp 252-254ċ; IR (KBr): 3300, 3050, 2800, 1700, 1600, 1522, 1475, 1250, 875, 800,750 cm-1; 1H NMR(CDCl3, 400 MHz): δ 7.69-7.71 (d, 1H, CO-CH=CH), 7.71-7.69 (d, J=8Hz), 7.27-8.29 (m, 8H, ArH), 8.01-7.99 (d, J=8Hz), 8.65 (s, 1H, NH).
IVb: Yellow solid, yield 68%, mp 242-244ċ; IR (KBr): 3350, 3100, 2800, 1700, 1600, 1550, 1475, 1350, 775, 700 cm-1; 1H NMR(CDCl3, 400 MHz): δ 2.76 (s, 3H, CH3), 7.48-7.50 (d, 1H, CO-CH=CH), 7.50-7.48 (d, J=8Hz), 7.27-7.78 (m, 8H, ArH), 7.70-7.68 (d, J=8Hz), 8.65 (s, 1H, NH).
IVc: Yellow solid, yield 72%, mp 248-250ċ; IR (KBr): 3300, 3050, 2800, 1700, 1600, 1500, 1450, 1250, 775, 700 cm-1; 1H NMR(CDCl3, 400 MHz): δ 3.91 (s, 3H, OCH3), 7.482-7.487 (d, 1H, CO-CH=CH), 7.487-7.481 (d, J=2.4Hz), 7.16-7.94 (m, 8H, ArH), 7.94-7.91 (d, J=12Hz), 8.59 (s, 1H, NH).
General procedure for the synthesis of pyrimidines of 6-chlorobenzimidazoles (Va)
To a refluxing mixture of chalcone (3.68g, 0.01mol) and guanidine nitrate (1.80g, 0.01mol) in ethanol (25ml) was added an aqueous solution of sodium hydroxide (40%, 5ml) portion wise during a period of 3 hours. Refluxing was continued further for 7 hours. The solvent was made to half of its volume. And on cooling, the solid product was separated out. Further, it was filtered, washed with cold aqueous ethanol followed by water and dried. Pure product was obtained by recrystallization from absolute ethanol.
Pyrimidine derivatives of 6-chlorobenzimidazoles (Va-k)
Va : Yellow solid, yield 60%, mp 83-85ċ; IR (KBr): 3335, 3194, 1602, 1543, 1442, 1253, 825, 715 cm-1; 1H NMR(CDCl3, 400 MHz): δ 4.72 (s, 2H, NCH2), 7.26-7.74 (d, J=12Hz), 7.26-7.74 (m, 8H, Ar-H), 7.72-7.70 (d, J=8Hz), 7.84 (s, 1H, NH).
Vb: Yellow solid, yield 48%, mp 80-82ċ; IR (KBr): 3327, 3196, 1620, 1543, 1425, 1246, 763, 705cm-1; 1H NMR(CDCl3, 400 MHz): δ 1.25 (s, 3H, CH3), 4.73 (s, 2H, NCH2), 7.19-7.21 (d, J=8Hz), 7.19-7.49 (m, 8H, Ar-H), 7.49-7.47 (d, J=8Hz), 7.88 (s, 1H, NH).
Vh : Yellow solid, yield 57%, mp 87-89ċ; IR (KBr): 3331, 3196, 1622, 1545, 1442, 1222, 812, 775 cm-1; 1H NMR(CDCl3, 400 MHz): δ 3.95 (s, 3H, OCH3), 4.72 (s, 2H, NCH2), 6.94-6.92 (d, J=8Hz), 6.88-7.68 (m, 8H, Ar-H), 7.54-7.52 (d, J=8Hz), 7.86 (s, 1H, NH).
Vi : Yellow solid, yield 52%, mp 82-84ċ; IR (KBr): 3198, 3055, 1616, 1577, 1425, 1336, 1246, 763 cm-1; 1H NMR(CDCl3, 400 MHz): δ 1.25 (s, 3H, CH3), 4.75 (s, 2H, NCH2), 7.22-7.20 (d, J=8Hz), 7.19-7.55 (m, 8H, Ar-H), 7.50-7.48 (d, J=8Hz), 7.90 (s, 1H, NH).
Results and Discussion
Scheme summarizes the synthetic route to obtain 6-chloro-2-(α-hydroxyethyl) benzimidazole (II) by reacting 4-chloro-O-phenylenediamine (I) with lactic acid. This on oxidation gives 6-chloro-2-acetylbenzimidazole (III). Further condensation with different aromatic / heteroaromatic aldehydes gives chalcone derivatives of 6-chlorobenzimidazoles (IVa-k). These react with guanidine nitrate in ethanol and aqueous solution of sodium hydroxide gives the title products (Va-k).
The IR spectrum of IV b showed characteristic absorption peaks at 3350 (NH), 1600 (C=O) and 1550 cm-1 (C=N). The 1H NMR spectrum of IVb displayed a singlet was noticed at δ 2.76, which was assigned to the CH3 protons. A multiplet between δ 7.27-7.78 (8H) accounted for aromatic protons. One doublet was observed at δ 7.48-7.50 accounted for (CO-CH=CH) of chalcone moiety.
The IR spectrum of Vb showed characteristic absorption peaks at 3327 (NH), 1620 (C=O) and 1543 cm-1 (C=N). The 1H NMR spectrum of Vb displayed a singlet for CH3 proton was observed at δ 1.25. A singlet of NCH2 protons of pyrimidine nucleus was observed at δ 4.73. A multiplet of aromatic protons was obtained from 7.19-7.49 and one NH at 7.88.
Similarly, the structures of the remaining compounds were confirmed by the spectral data. Further, they have been tested for antimicrobial activity.
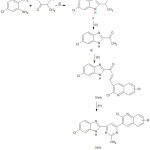 |
Scheme 1: R= (a)-6-Cl; (b)-6-CH3; (c)-6-OCH3; (d)-6-Br; (e)-6-F; (f)-7-Cl; (g)-7-CH3; (h)-7-Meo; (i)-8-CH3; (j)-8-OCH3; (k)-H. Click here to View scheme |
R= (a)-6-Cl; (b)-6-CH3; (c)-6-Meo; (d)-6-Br; (e)-6-F; (f)- 7-Cl; (g)-7-CH3; (h)-7-Meo; (i)-8-CH3; (j)- 8-Meo; (k)-H.
Reagents and Conditions
(i) lactic acid, 4N HCl, MW irradiation 190 minutes (ii) K2Cr2O7, H2SO4 (25% v/v) 2h, (iii) 10% NaOH, Quinoline-3-carbaldehydes, ethanol, 0.5 h, (iv) Guanidine nitrate, ethanol, NaOH (40%), for 10h.
Table 1: Physical Characterization of synthesized compounds (Va-k)
|
Sl.No |
Compoud Code |
R |
Molecular Formula |
M.P Ċ |
Molecular Weight |
N% |
H% |
C% |
Cl% |
|
1 |
Va |
6-Cl |
C20H11Cl3N6 |
84 |
441.70 |
19.03 |
2.51 |
54.38 |
24.08 |
|
2 |
Vb |
6-CH3 |
C21H14Cl2N6 |
87 |
421.28 |
19.95 |
3.35 |
59.87 |
16.83 |
|
3 |
Vc |
OCH3 |
C21H14Cl2N6O |
87 |
437.28 |
19.22 |
3.23 |
57.68 |
16.22 |
|
4 |
Vd |
6-Br |
C20H11BrCl2N6 |
92 |
486.15 |
17.29 |
2.28 |
49.41 |
14.59 |
|
5 |
Ve |
6-F |
C20H11FCl2N6 |
94 |
425.25 |
19.76 |
2.61 |
56.49 |
16.67 |
|
6 |
Vf |
7-Cl |
C20H11Cl3N6 |
85 |
441.70 |
19.03 |
2.51 |
54.38 |
24.08 |
|
7 |
Vg |
7-CH3 |
C21H14Cl2N6 |
86 |
421.28 |
19.95 |
3.35 |
59.87 |
16.83 |
|
8 |
Vh |
OCH3 |
C21H14Cl2N6O |
89 |
437.28 |
19.22 |
3.23 |
57.68 |
16.22 |
|
9 |
Vi |
8-CH3 |
C21H14Cl2N6 |
88 |
421.28 |
19.95 |
3.35 |
59.87 |
16.83 |
|
10 |
Vj |
OCH3 |
C21H14Cl2N6O |
85 |
437.28 |
19.22 |
3.23 |
57.68 |
16.22 |
|
11 |
Vk |
H |
C20H12Cl2N6 |
85 |
407.25 |
20.64 |
2.97 |
58.98 |
17.41 |
Biological Evaluation20-25 Antibacterial Activity
The synthesized products have been tested for antibacterial activity against two Gram-positive bacteria viz., Bacillus subtilis, Staphylococcus aureus, and two Gram-negative bacteria viz., Proteus Mirabilis and Escheria coli. The standard drug used was Ciprofloxacin and DMSO as a solvent. Test products and the standard drug were used at a concentration 100µg/ml and 50µg/ml. The zones of inhibition of compounds were recorded after incubation of 24 hours at 37oC.
The results of the antibacterial activity reveals that, the products Va, Vb, Vd and Vf displayed relatively high antibacterial activity, while products Ve, Vg, Vh and Vi showed reasonable antibacterial activity. The remaining products showed low activity.
Antifungal Activity
The antifungal activity of the products has been screened against two fungi viz., Aspergilus Niger and Candida albicans by cup-plate method. The standard drug used was Fluconazole and DMSO as a solvent. Test products and standard drug were used at a concentration of 100µg/ml and 50µg/ml. The zones of inhibition of compounds were recorded after incubation of 48 hours at 25oC.
Further from antifungal activity results, products Va, Vb, Vd and Vf showed excellent results for antifungal activity, while the products Ve, Vg, Vh and Vi also showed high antifungal activity. The remaining products exhibited low activity.
Table 2: Antibacterial and antifungal activity of synthesized compounds (Va-k)
| Compound |
Antibacterial activitya,b |
Antifungal activiya,b |
||||
| B. subtilis | S. aureus | P.mirabilis | E. coli | A. niger | C. albicans | |
|
Va |
25/21 |
24/20 |
22/21 |
23/20 |
17/15 |
19/16 |
|
Vb |
23/21 |
25/24 |
22/20 |
24/23 |
17/15 |
18/16 |
|
Vc |
09/06 |
10/08 |
06/05 |
07/05 |
08/05 |
06/04 |
|
Vd |
24/22 |
23/20 |
22/20 |
25/23 |
17/15 |
18/15 |
|
Ve |
18/16 |
17/14 |
14/13 |
15/14 |
13/11 |
14/12 |
|
Vf |
23/21 |
22/19 |
24/22 |
22/20 |
18/16 |
17/15 |
|
Vg |
18/15 |
19/16 |
17/15 |
18/16 |
12/11 |
14/12 |
|
Vh |
18/16 |
17/15 |
16/14 |
19/17 |
13/10 |
12/11 |
|
Vi |
17/16 |
17/15 |
19/17 |
18/16 |
14/13 |
13/11 |
|
Vj |
07/05 |
08/05 |
10/07 |
09/06 |
06/03 |
07/04 |
|
Vk |
07/05 |
09/07 |
06/05 |
08/06 |
07/05 |
06/03 |
|
Ciprofloxacinb |
26 |
26 |
26 |
26 |
– |
– |
|
Fluconazoleb |
– |
– |
– |
– |
23 |
23 |
aZone of inhibition at 100µg/ml.
bZone of inhibition at 50µg/ml.
Minimum inhibitory concentration was found at 40µg/ml concentration.
Conclusion
A simple protocol for the synthesis of some new pyrimidines of 6-chlorobenzimidazoles under synthetic microwave irradiation method and also by the conventional method. It was noted that the preferred synthetic route does an excellent yield and shortened reaction time for benzimidazole synthesis. The synthesized compounds were screened for their antibacterial and antifungal activities. Many of these products showed significant activity.
Acknowledgement
The authors are grateful to Vision Group on Science and Technology (VGST), Government of Karnataka state for financial support.
References
- Salahuddin.; Shaharyar, M.; Mazumdar, A. Arab. J. of chem. 2017, 10 (1), 157-173.
- Singh R. M.; Srivastava, A. Ind. J. of Chem. 2005, 44 (B), 1868-1875.
- Sawhney,S.N.; Vir, D.; Gupta, A. Ind. J. of Chem. 1990, 29 (B), 1107-1112.
- Bhalgat, C. M.; Ramesh, B. Bulletin of Fac. of Pharm. Cairo University. 2014, 52, 259–267.
CrossRef - Swaminathan, S.; Ingarsal, N. Orient. J. of Chem. 2018, 34 (2), 777-782.
CrossRef - Adhikari, A.; Kalluraya, B.; Sujith, K.V.; Gouthamchandra.; Mahmood, R.; Saud. Pharm. J. 2012, 20, 75–79.
CrossRef - Bahashwan, S. A.; Fayed, A. A.; Abd El-Galil, E. A.; Eman, M. F.; Kalmouch, A. Molecules. 2013, 18, 15051-15063.
CrossRef - Antre, R. V.; Cendilkumar, A.; Goli, D.; Andhale, G. S.; Oswal, R. J. Saud. Pharma. J. 2011, 19, 233–243.
CrossRef - Al-Issa, S. A. Saud Pharm. J. 2013, 21, 305–31.
CrossRef - Shaaban, M. R.; Saleh, T. S.; Farag, A. M. Heterocycl. 2007, 71 (8), 1765-1777.
CrossRef - Abdelhamid, A. O.; Eman, K. A.; Nadia A. A.; Riheem, A.; Ahmed, S. A. Phosph. Sulf. And Silicon and the Relat. Element. 2010, 185 (4), 709-718.
- Narwal, S.; Kumar S.; Verma, P. K.; Chem. Cent. J. 2017, 11 (52), 1-9.
- Wang, Z. Compreh. Org. Name React. And Reag. 2010, 496, 2197-2199.
- Reddy, V. M.; Reddy, K. R. Chine. Chem. Lett. 2010, 21, 1145-1148.
CrossRef - Mamedov, I. G.; Mamedov, Y. V.; Khrustalev, V. N.; Bayramov, M. R.; Maharramov, A.M. Ind. J. of Chem. 2017, 56 (B), 192-196.
- Kachroo, M.; Panda, R.; Yadav, R. Der. Pharm. Chemic. 2014, 6 (2), 352-359.
- Sharmila, A. G.; Shivakumar, B.; Gaviraj, E. N. Der. Pharm. Chemic. 2016, 8 (5),33-37.
- Tabassum, S.; Suresha Kumara, T. H.; Jasinski, J.P.; Millikan, S. N.; Yathirajan, H. S.; Sujan Ganapathy, P.S.; Sowmya, H. B. V.; More, S. S.; Nagendrappa, G.; Kaur, M.; Jose. G. J. of Molecul. Struct. 2014, 1070, 10-20.
CrossRef - Rajakumar, P.; Raja, R. Tetrahed. Lett. 2010, 51, 4365-4370.
CrossRef - Vaidehi, B. N. B.; Gnana Deepika, K.; Satya, R. V.; Bangaramma, R. R.; Harish Kumar, R.; Ratna Sudha, Y.; Ravi Kumar, T. Inter. J. of Res. in Pharm. And Chem. 2012, 2 (2), 322-326.
- Venkatesan, P.; Sumathi, S. J. of Heterocycl. Chem. 2010, 47, 81-84.
- Ramanpreet, W.; Hedaitullah, M. D.; Syeda, F. N.; Khalid I.; Lambar, H.S. Inter. J of Res. in Pharm. And Chem. 2011, 1 (3), 565-574.
- Radha, Y.; Manjula, A.; Madhav Reddy, B.; Vitthal Rao, B. Ind. J. of Chem. 2011, 50 (B), 1762-1773.
- Kalirajan, R.; Leela, R.; Jubie, S.; Gowramma, B.; Gomathy, S.; Sankar, S. Ind. J. of Chem. 2011, 50 (B), 1794-1799.
- Liu M.; Sun, X.; Zhang, J. Arab. J. of Chem. 2017, 10 (2), 167-171.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









