Mechanical Properties and Antibacterial Activity of Cellulose Composite Based Coconut Water with Addition Glycerol, Chitosan, and Silver Nanoparticle
1Department of Chemistry Education, FMIPA, Yogyakarta State of University, Jl. Colombo No. 1, Sleman DIY 55281 Indonesia.
2Department of Biology Education, FMIPA, Yogyakarta State of University, Jl. Colombo No. 1, Sleman DIY 55281 Indonesia.
Corresponding Author E-mail: eli_rohaeti@uny.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340320
Article Received on : January 03, 2018
Article Accepted on : April 13, 2018
Article Published : 05 May 2018
This study aims to study mechanical properties and antibacterial activity of celluloses and theirs composites. The cellulose was prepared from coconut water fermented by Acetobacter xylinum. Cellulose was composited with chitosan and glycerol by immersion method and the addition of silver nanoparticles. Preparation of silver nanoparticles performed by chemical reduction of silver nitrate solution and polyvinyl alcohol as a stabilizer. UV-Vis spectroscopy was used to determine the formation of silver nanoparticles. Characterization of composites included observation of physical properties, mechanical properties, as well as antibacterial tests against S. aureus, E. coli, and C. albicans. The addition of glycerol can increase elongation at break of composites. The addition of glycerol and chitosan can decrease elongation at break and strength at break of the composites. The composite of cellulose - glycerol - chitosan - silver nanoparticles shows the highest antimicrobial activity against S. aureus on 24 hours of incubation, whereas composite of cellulose - glycerol - silver nanoparticles shows the highest antimicrobial activity against E. coli and C. albicans.
KEYWORDS:coconut water; antibacterial activity; glycerol; chitosan; cellulose
Download this article as:| Copy the following to cite this article: Rohaeti E, Fx E. W. L, Rakhmawati A. Mechanical Properties and Antibacterial Activity of Cellulose Composite Based Coconut Water with Addition Glycerol, Chitosan, and Silver Nanoparticle. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Rohaeti E, Fx E. W. L, Rakhmawati A. Mechanical Properties and Antibacterial Activity of Cellulose Composite Based Coconut Water with Addition Glycerol, Chitosan, and Silver Nanoparticle. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=45406 |
Introduction
Cellulose is an unbranched homopolysaccharide, elastic fibres, and insoluble in water. The cellulose is found in the protective cell wall, especially in the stems, branches and all woody parts of plant tissue, besides cellulose can be made with the help of Acetobacter xylinum1. In a liquid medium containing carbohydrates, this bacterium will produce acids of vinegar and a white coating on the surface of liquid media called nata.
One source of carbohydrates can be obtained from household waste, including coconut water. The utilization of coconut water has not been done optimally. In line with the increasing human need to make use of coconut water into functional materials with better economic value. When viewed further, waste coconut water has nutrients that can still be utilized. Coconut water contains 95.50% of water, 6.60% of potassium, 2.80% of total sugar,0.80% of reducing sugar and 0.62% of ash. Coconut water also contains sucrose and vitamins B complex including nicotinic acid, pantothenic acid, biotin, and folic acid.2 These nutrients can be used for the growth of Acetobacter xylinum, especially carbon and nitrogen content. Acetobacter xylinum can break down the sugar component in the coconut water medium and is capable of forming a polysaccharide known as cellulose.
The Acetobacter xylinum is capable of forming a cellulose-forming coating layer that can reach a few centimetres thick.3 Bacterial cellulose has the characteristic similar to human skin, so good for the treatment of burns on the human skin, especially in preventing infections caused by pathogenic microbes, but bacterial cellulose easily absorbs fluid (hygroscopic) so easily attacked by microbes. The bacterial cellulose can be modified by the addition of an ingredient into the culture medium to improve its antibacterial properties3. Modifications can be made through the addition of glycerol, chitosan, and silver nanoparticles. The addition of glycerol in the manufacture of bacterial cellulose in general aims to improve the mechanical properties of cellulose especially the elongation at break. 4, 5 Chitosan in the form of a solution and gel, can be used as bacteriostatic, fungistatic, and coating material. The superior properties of bacterial cellulose and chitosan can be a composite material that interacts between the chitosan molecule part (glucosamine unit and N-acetylglucosamine) in chitosan with the resulting cellulose chain. The combination of bacterial cellulose and chitosan can improve the antibacterial properties of cellulose.6 Modification of composite of bacterial cellulose – glycerol – chitosan can be done by adding nanometer – sized silver. Silver in the form of nanoparticles can interact among cell molecules contained in small organisms such as in bacteria or yeasts through electrostatic interactions.
Silver has a wide spectrum of antimicrobial activity that exhibits low toxicity to mammalian cells. Silver nanoparticles are generally smaller than 100 nm and contain as much as 20-15,000 atoms of silver. The silver nanoparticle has physical, chemical and biological properties.7 The antimicrobial activity of its silver and composite nanoparticles can be tested against Staphylococcus aureusand Escherichia coli, as well as Candida albicans yeast. Staphylococcus aureus is a major pathogen in humans. Almost everyone has experienced various S. aureus infections during their lifetime, such as food poisoning or mild skin infections, to incurable infections.8 Candida albicans includes endogenous or exogenous yeasts that are generally present in water, soil, and air, may cause opportunistic mycosis. As many as 70% of candida infections in humans are caused by Candida albicans, the rest are caused by C. tropicalis, C. parapsilosis, C. guillermondii, C. kruzei.8
Based on the background, it is necessary to make various modifications to the cellulose from coconut water in inhibiting the growth of Staphylococcus aureus, Esherichia coli, and Candida albicans yeast. Modifications were made by adding glycerol, chitosan, and silver nanoparticles. Characterization includes the physical properties of cellulose and its composites, and the test of physical, mechanical, and antibacterial activities. This study aims to prepare a coconut water-based cellulose composite, test the mechanical properties, as well as antibacterial activity of cellulose and its composites.
Methods
Tools and Materials
The tools used in the study include UV-Vis spectrophotometer (Shimadzu 1601, Japan), tensile tester, oven (CIKA), autoclave (ALP Co., Ltd. KT-40 model), Bunsen burner, magnetic stirrer, hot plate, digital camera, sliding wheel, petri dish, micropipette, scales, three-neck flask, reflux set, and other glassware.
Materials used in the study include coconut waste (clear color, coconut scent, odorless, and non-greasy), chitosan, glycerol, starter of Acetobacter xylinum bacteria (PT Chemix Pratama), yeast strains of Candida albicans ATCC 10231 (Pathology Laboratory in UGM), Staphylococcus aureus ATCC 25923 strain and Escherichia coli ATCC 32518 (Microbiology Laboratory of Yogyakarta Health Center), HCl, NaOH, acetic acid, silver nitrate (E-Merck), sodium citrate (E-Merck), potato dextrose agar medium ), Nutrient Broth (NB) medium (Oxoid) medium, Nutrient Agar (NA) medium (Oxoid), urea, granulated sugar, and alcohol.
Preparation of Cellulose and its Composite
As much as 100 mL of wastewater of coconut is poured into Erlenmeyer which has been equipped with a magnetic stirrer, then added 10 grams of sugar and 0.5 grams of urea, and stirred until dissolved. The mixture was acidified with the addition of 25% CH3COOH until pH = 4. The solution in the container was allowed to reach temperature 40 – 60°C aseptically, then added 20 mL of Acetobacter xylinum. The culture was incubated for 7 days at room temperature. The formed layers were washed with aquadest to remove the residue of the culture medium. After purification of the pellicle by immersion in 3% of NaOH for 12 hours by repeating 3 times the process to dissolve bacterial and pyrogenic cells, that bacterial cellulose samples were immersed in 3% of HCl solution to neutralize and washed again with aquadest. Furthermore, drying of bacterial cellulose samples at 70 – 800C for ± 120 minutes to obtain cellulose product (S).
Furthermore, the composite of cellulose – glycerol (CG) was prepared by pouring 100 mL of waste water into Erlenmeyer then adding 10 grams of sugar and 0.5 grams of urea, and stirring until dissolved. The mixture was acidified by adding 25% of CH3COOH until pH = 4. Then 0.5 g (1.2 mL) of glycerol as a plasticizer, was added and stirred while heated. It was then poured hot in a sterilized and closed fermented container. The solution in the container was allowed to reach 40 – 60°C aseptically, then 20 mL of Acetobacter xylinum was added. The culture was incubated for 12 days at room temperature. The formed layers were washed with aquabidest to remove the residue of the culture medium. After purification of the pellicle by immersion in 3% of NaOH for 12 hours by repeating 3 times the process to dissolve bacterial and pyrogenic cells, the solution was then filtered to remove the dissolved material. After that the pellicle was soaked in 3% of HCl solution to neutralize and be washed again with aquadest. Furthermore, drying of the samples at 70 – 80°C for ± 120 minutes.
Furthermore, the composite of cellulose – glycerol – chitosan (CGK) was produced by immersing the CG composite in 2% of chitosan solution until the solution of chitosan was absorbed. Then the sample was washed with 500 mL of 1 M NaOH and aquadest to remove the remaining alkali and neutralize the sample. The sample is then dried at a temperature between 50-65°C for 24 hours. The cellulose and its composite were characterized their physical and mechanical properties.
Preparation of Silver Nanoparticle
The silver nanoparticles were prepared by refluxing 100 mL of a 1 x 10-3 M silver nitrate solution by streaming nitrogen gas at a temperature of less than 90°C while stirring. Then add drops of sodium citrate at a temperature of about 80-90°C to a pale yellow solution. The heating and gas flow of nitrogen was stopped while stirring was carried out until the room temperature reached. The resulting colloid was characterized by a UV-Vis spectrophotometer.
Application of silver nanoparticle to cellulose and its composite
The application of silver nanoparticles to cellulose, cellulose-glycerol composites, and cellulose – glycerol-chitosan composites was performed by introducing cellulose and its composite into colloidal silver nanoparticles until submerged and then shakered for 60 minutes at 145 rpm. Table. 1 shows the variation of cellulose and its composite were prepared in this work.
Methods
Tools and materials
The tools used in the study include UV-Vis spectrophotometer (Shimadzu 1601, Japan), tensile tester, oven (CIKA), autoclave (ALP Co., Ltd. KT-40 model), Bunsen burner, magnetic stirrer, hot plate, digital camera, sliding wheel, petri dish, micropipette, scales, three-neck flask, reflux set, and other glassware.
Materials used in the study include coconut waste (clear color, coconut scent, odorless, and non-greasy), chitosan, glycerol, starter of Acetobacter xylinum bacteria (PT Chemix Pratama), yeast strains of Candida albicans ATCC 10231 (Pathology Laboratory in UGM), Staphylococcus aureus ATCC 25923 strain and Escherichia coli ATCC 32518 (Microbiology Laboratory of Yogyakarta Health Center), HCl, NaOH, acetic acid, silver nitrate (E-Merck), sodium citrate (E-Merck), potato dextrose agar medium ), Nutrient Broth (Oxoid) medium, Nutrient Agar (NA) medium (Oxoid), urea, granulated sugar, and alcohol.
Preparation of cellulose and its composite
As much as 100 mL of wastewater of coconut is poured into Erlenmeyer which has been equipped with a magnetic stirrer, then added 10 grams of sugar and 0.5 grams of urea, and stirred until dissolved. The mixture was acidified with the addition of 25% CH3COOH until pH = 4. The solution in the container was allowed to reach temperature 40 – 600C aseptically, then added 20 mL of Acetobacter xylinum. The culture was incubated for 7 days at room temperature. The formed layers were washed with aquadest to remove the residue of the culture medium. After purification of the pellicle by immersion in 3% of NaOH for 12 hours by repeating 3 times the process to dissolve bacterial and pyrogenic cells, that bacterial cellulose samples were immersed in 3% of HCl solution to neutralize and washed again with aquadest. Furthermore, drying of bacterial cellulose samples at 70 – 80°C for ± 120 minutes to obtain cellulose product (S).
Furthermore, the composite of cellulose – glycerol (CG) was prepared by pouring 100 mL of waste water into Erlenmeyer then adding 10 grams of sugar and 0.5 grams of urea, and stirring until dissolved. The mixture was acidified by adding 25% of CH3COOH until pH = 4. Then 0.5 g (1.2 mL) of glycerol as a plasticizer, was added and stirred while heated. It was then poured hot in a sterilized and closed fermented container. The solution in the container was allowed to reach 40 – 60°C aseptically, then 20 mL of Acetobacter xylinum was added. The culture was incubated for 12 days at room temperature. The formed layers were washed with aquabidest to remove the residue of the culture medium. After purification of the pellicle by immersion in 3% of NaOH for 12 hours by repeating 3 times the process to dissolve bacterial and pyrogenic cells, the solution was then filtered to remove the dissolved material. After that the pellicle was soaked in 3% of HCl solution to neutralize and be washed again with aquadest. Furthermore, drying of the samples at 70 – 80°C for ± 120 minutes.
Furthermore, the composite of cellulose – glycerol – chitosan (SGK) was produced by immersing the CG composite in 2% of chitosan solution until the solution of chitosan was absorbed. Then the sample was washed with 500 mL of 1 M NaOH and aquadest to remove the remaining alkali and neutralize the sample. The sample is then dried at a temperature between 50-65°C for 24 hours. The cellulose and its composite were characterized their physical and mechanical properties.
Preparation of silver nanoparticle
The silver nanoparticles were prepared by refluxing 100 mL of a 1 x 10-3 M silver nitrate solution by streaming nitrogen gas at a temperature of less than 90°C while stirring. Then add drops of sodium citrate at a temperature of about 80-90°C to a pale yellow solution. The heating and gas flow of nitrogen was stopped while stirring was carried out until the room temperature reached. The resulting colloid was characterized by a UV-Vis spectrophotometer.
Application of silver nanoparticle to cellulose and its composite
The application of silver nanoparticles to cellulose, cellulose-glycerol composites, and cellulose – glycerol-chitosan composites was performed by introducing cellulose and its composite into colloidal silver nanoparticles until submerged and then shakered for 60 minutes at 145 rpm. Table. 1 shows the variation of cellulose and its composite were prepared in this work.
Table 1: Variation of cellulose and its composite
| No. | Code of samples | Component |
| 1 | S | Cellulose |
| 2 | CG | Cellulose and glycerol |
| 3 | SGK | Cellulose, glycerol, and chitosan |
| 4 | SN | Cellulose and silver nanoparticle |
| 5 | SGN | Cellulose, glycerol, and silver nanoparticle |
| 6 | SGKN | Cellulose, glycerol, chitosan, and silver nanoparticle |
Test of Antibacterial Activity
The test microbes were cultured on NB media for Staphylococcus aureus and GDP media for Candida albicans for 24 hours at 37°C. The turbidity of test bacteria using a scale of turbidity (OD) of 1 or Brown III is 108 CFU/mL. As much as 15 mL of NA medium with a temperature of ± 500C was poured into petridish. After the media solidified, the Staphylococcus aureus was poured and flattened in a way evenly applied. Then a cellulose sample was planted on prepared media and incubated for 2 x 24 hours at 37°C. Clear zones indicated a growth barrier of microorganisms by antimicrobial agents on the surface of the agar medium.9
As much as 15 mL of PDA media with temperature ± 500C was poured into a petridish. After the media solidified, the Candida albicans was poured and flattened in an evenly applied manner and the samples were planted in a prepared media and incubated for 2 x 24 hours at 37°C.The diameter of inhibition zone was measured by 3 times at different positions, the measurement was taken perpendicularly from the three zones of the clear zone side through the sample diameter. Data of inhibition zone was analyzed using a statistic analysis One Way ANOVA with significance level (P <0.05). If there was a difference, then proceed with an analysis of Duncan Multi-Range Test (DMRT).
Results and Discussion
Physical Properties of Cellulose and its Composite
The physical properties of cellulose and its composites are shown in Table. 2. The addition of glycerol and chitosan to cellulose causes a decrease in the resultant wet mass of the composite, indicating that the addition of glycerol and chitosan affects the formation of the pellicle. Glycerol has hydrophilic properties so that the water content in the CG sample increases and the resulting water content becomes high. Decreasing of wet mass of composites due to addition of glycerol and chitosan, this suggests the possibility of interaction between the -OH group of the cellulose with -NH group of the chitosan and -OH group of the glycerol producing a lower-mass molecule. The SGK composite has a higher dry yield than S and CG. The cellulose is a very hygroscopic material and attracts water through hydrogen interactions.10 This is consistent with the results in the S and CG groups having a smaller dry mass than SGK. The event can be explained because the added chitosan is able to enter the cellulose pores and coat the cellulose surface so that the water in the environment can not enter. Observation of other physical properties of colour change occurs in SGK, this is influenced by chitosan added has a brownish-yellow colour and has a strong acid odour. Based on the physical properties of cellulose samples and their variations, it can be seen that all samples are transparent. Organoleptic observation of color changes in SGK, the addition of chitosan, causing the color to be brownish-yellow, it is because chitosan has a brownish yellow color when dissolved in acetic acid and can cause acid odor.
Table 2: Physical properties of cellulose and its composites
| Parameter | S | CG | SGK |
| Wet mass | 100.44 g | 106.33 g | 94.70 g |
| Dry mass | 1.40 g | 1.49 g | 3.56 g |
| % wet yield | 83.7 % | 88.60 % | 78.91% |
| % dry yield | 1.39 % | 1.40 % | 3.75% |
| Transparency | Transparent | Transparent | Transparent |
| Colour | White | Yellow | Brownish yellow |
| Elasticity | Elastic | Elastic | Less elastic |
| Odour | Odourless | Odourless | The sour-smelling |
Characteristic of Silver Nanoparticle
Fig. 1 shows the UV-Vis spectrum of the of silver nanoparticles. The silver nanoparticles successfully prepared from AgNO3 are shown by an absorption peak of 0.954 at a wavelength of 421.80 nm.The size of the established silver nanoparticles can be predicted based on the λmax value, the larger the particle size of the silver, the absorption peak will shift towards a larger wavelength in the 395-450 nm range and the wider peak. These results indicate that the preparation of silver nanoparticles is perfect. Based on the results of the nanoparticle size test and compared with the previous work11, it was demonstrated that the silver nanoparticles were produced by the reduction of silver nitrate solution with a particle size of 60-80 nm.
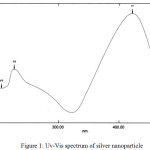 |
Figure 1: Uv-Vis spectrum of silver nanoparticle |
Mechanical Properties of Cellulose and its Composites
The mechanical properties of the cellulose and composite samples after addition of the silver nanoparticles are shown in Table. 3. The addition of glycerol to cellulose enhances the extent of cellulosic breakdown. The addition of glycerol to increase the number of oxygen atoms that have free electron pairs leads to increased flexibility of the group, the ability of the polymer undergoes an increasing extension.10, 12 Glycerol acts as an internal plasticizer that can increase the elongation at break of the polymer.13 The decrease in break strength in SGN and SGKN samples due to the properties of glycerol as the plasticizer to make the rigidity of the material decreases, The mechanical properties of cellulose can be explained by the intensity of the functional groups contained in each cellulose. The cellulose has many OH groups, can increase flexible groups, so the elasticity of the polymer increases.
Table 3: Mechanical properties of cellulose and its composites with addition silver nanoparticle
| No. | Type of sample | Parameter of mechanical properties | |
| Strength at break (MPa) | Elongation at break (%) | ||
| 1. | SN | 40.67 | 19.81 |
| 2. | SGN | 31.75 | 30.65 |
| 3. | SGKN | 3.74 | 2.13 |
The addition of chitosan in the SGKN decreases the value of strength at break and elongation, this may be due to the decreasing of the intermolecular bonding distance (Li et al., 2015) thereby reducing the mobility of SGKN molecules. The decreasing strength at break in SGKN could be caused by crystallinity in composite by the addition of amorphous chitosan.5 The addition of glycerol and chitosan to the SGKN composite decreases the elongation at break, this may be due to the intermolecular bonding of cellulose. The addition of glycerol and chitosan can increase the amount of hydrogen bonding in SGKNs so that molecular mobility of those decreases. In addition, the amorphous chitosan and the presence of hydrogen bonds between the -OH group of glycerol and the -NH2group of chitosan with the -OH group of cellulose and the intramolecular bond between the chitosan molecules14 caused decreasing the value of elongation at break of the SGKN composite.
Antibacterial Activity of Cellulose and its Composites
Analysis of antimicrobial activity from SN, SGN, and SGKN samples against test microbes i.e. Staphylococcus aureus, Escherichia coli, and Candida albicans for 24 hours was shown in Table. 4. The results showed that all samples of composites by addition silver nanoparticles have an antimicrobial effect.
Table 4: Diameter of inhibition zone of cellulose and its composites against Staphylococcus aureus, Escherichia coli, and Candida albicans for 24 hours
| No | Cellulose | Diameter of inhibition zone (mm) | ||
| S.aureus | E.coli | C. albicans | ||
| 1. | SN | 1.750 | 0.830 | 1.550 |
| 2. | SGN | 1.910 | 1.660 | 2.290 |
| 3. | SGKN | 2.320 | 1.330 | 1.620 |
The SGKN samples show the highest diameter of the zones against S. aureus compared to other composites and the SGN samples show the highest clear zone against E. coli and C. albicans compared to other composite samples. This means that the SGKN sample has an antibacterial activity or the ability to inhibit S. aureus better than the SN and SGN. This is probably due to the presence of bonds between the silver nanoparticles and the -OH group of glycerol and the bonds between the silver nanoparticles and the -NH2 groups of chitosan. The SGKN contains more -OH and -NH2 groups than SN and SGN samples. The antibacterial properties become larger by the increasing number of interactions that occur between the silver nanoparticles with those clusters and the presence of free –OH groups as well as -NH which can interact with the microbial cell wall components. The interaction between cellulose with glycerol and chitosan is presented in Fig. 2. In addition, the antibacterial activity of SGKN can be caused by the ability of chitosan as a bactericidal agent in killing bacteria15, 16, 17.
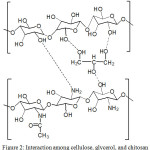 |
Figure 2: Interaction among cellulose, glycerol, and chitosan |
The SGN sample shows the highest antibacterial activity against E.coli and C. albicans compared to other composite samples. The addition of glycerol to cellulose increases the number of oxygen atoms that have free electron pairs so that the interaction with the silver nanoparticles becomes larger. Figure 3 shows the interaction between cellulose and glycerol. The composite of cellulose – glycerol has free -OH more to bind to the silver nanoparticles so that the activity against bacteria is higher. The increase of flexible groups in composite causes silver nanoparticles to bind more easily. In addition, glycerol has antibacterial properties.17, 18, 19
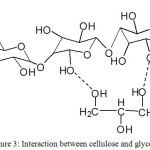 |
Figure 3: Interaction between cellulose and glycerol |
Cellulose has fewer OH groups than the other samples. This is due to the regular (crystalline) cellulose structures that make it difficult for the silver nanoparticles to bind to functional group of the cellulose, while the addition of chitosan results in the more amorphous nature of cellulose because of its decreased crystallinity value.5 This facilitates the electrostatic interaction of the silver nanoparticles attacking the functional groups in cellulose
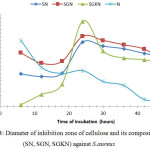 |
Figure 4: Diameter of inhibition zone of cellulose and its composites (SN, SGN, SGKN) against S.aureus |
Fig. 4. shows the antibacterial activity of SGKN, SGN, SN, and silver nanoparticle (N) against Staphylococcus aureusat every 6 hours. The largest inhibitory zone diameter in the SGN sample was followed by SN and SGKN. The low ability of SGKN to inhibit the activity of Staphylococcus aureus is caused by the interaction between chitosan and silver nanoparticle causing the decrease of intramolecular hydrogen bond strength and chitosan intermolecular after the existence of nanoparticle, and the formation of hydrogen bond between chitosan and glycerol. This was possible because the interaction of Ag with the -NH2 20 and -OH groups causes a decrease in inhibitory power to the Staphylococcus aureus.
There is a difference in inhibitory zone diameter of all cellulose samples on the growth of Staphylococcus aureus. All cellulose samples coated silver nanoparticles show higher antibacterial activity than silver nanoparticles, except SGKN samples in 6 to 18 hours of incubation. The SGKN samples show the highest antibacterial activity compared to other samples at incubation for 24 hours. This can be explained because in 24hours incubation, there is an interaction between silver nanoparticles with chitosan which can increase antibacterial activity12 also because chitosan shows bactericidal properties.16 In accordance with the previous results14 that modification of silver nanoparticles with chitosan/Ag/ZnO can improve the ability of Ag nanoparticles as inhibiting bacterial activity. The inhibition mechanism of chitosan against Staphylococcus aureus is due to the positive charge of chitosan derived from an ionic-bound amine (NH3+) group that is reactive to the surface of the bacterial cell membrane, this will cause the entire surface of the S. aureus cell membrane to be coated by chitosan, so that S. aureus can not contact with the outer environment of the cell. Furthermore, ionic bonds formed between chitosan and S. aureus cell membrane will disrupt the permeability of membranes and chitosan able to penetrate the membrane of S. aureus cells. Chitosan is brought into the intercellular space of S. aureus and binds to DNA of S. aureus because of its strong affinity with DNA of S. aureus, then interferes with mRNA and protein synthesis. Then there will be disruption of cell function, followed by leakage of cell protein because chitosan meet the intercellular space then cell protein depressed to intercellular space, followed by lysis of S. aureus and then S. aureus death.21
The ability of antimicrobial of each sample against S. aureus has difference and the SGN compocite has a larger inhibitory compared with other cellulose. This can be explained because the silver nanoparticles from SGN can interact with Staphylococcus aureus through the bacterial cell wall. This interaction causes the changing of permeability of the Staphylococcus aureus cell wall. The permeability of the Staphylococcus aureus cell wall to be disrupted. During the diffusion process, the silver nanoparticles move closer to the bacterial cell membrane and penetrate into the bacteria22. Bacteria membranes contain proteins with sulfur compounds as their main component.23 The interaction involves the interaction of nanoparticle with biological macromolecules, by releasing heavy metal ions that react with thiol (-SH) groups on surface proteins. Monovalent silver ions (Ag+) can replace hydrogen cations (H+) from the thiol sulfhidryl group, resulting in S-Ag groups and inactivating proteins, decreased membrane permeability, and ultimately leading to cellular death.7
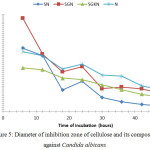 |
Figure 5: Diameter of inhibition zone of cellulose and its composites against Candida albicans |
Fig. 5. shows the greatest antibacterial activity in inhibiting the growth of Candida albicans is SGN sample followed by SGKN and SN. The emergence of the highest inhibition zones at the 6th hour of each sample can be due to the 6th hour is an effective time for silver nanoparticles in inhibiting Candida albicans. Inhibitory zones begin to appear at the beginning of the first 6 hours to the end of the 48th hour. This is because the ability of silver nanoparticles interferes with the integrity of Candida albicans cell plasma membranes. There were several intracellular components released during disruption of the plasma membrane by silver nanoparticles, so membrane permeability was impaired and lead to leakage of ions and other materials, thus possibly causing Candida albicans lysis and it death.17
The effect of silver nanoparticles on bacteria varied with the concentration of silver nanoparticles and the time of exposure.14 DNA of Candida albicans becomes thick after exposure for 6 hours and after 12 hours of exposure, cellular content releases due to cell wall damages. It is seen that at the 12th hour the average of the inhibition zone diameter of SN, SGN, SGKN, and silver nanoparticles decreases.
The antibacterial activity of cellulose and composite in inhibiting the growth of S. aureus and Candida albicans tends to increase again at 24 hours and down again at the 30th hour. The antibacterial activity of cellulose and its composites against the growth of S. aureus and C. albicans microbes in incubation for 30 hours shows a decrease in the diameter of the inhibition zone and the presence of spots of S. aureus and C. albicans around the test sample. Thus incubation longer than 48 hours will not significantly effect the reduction of antibacterial activity. Cellulose and its composites are able to act as bactericidal rather than bacteriostatic. The bactericidal is the ability of a compound to kill bacteria and bacteria will not have the ability to regenerate even if the compound is removed.
The inhibition of cellulose and its composites against S. aureus showed no significant difference with significance (P> 0.005), indicating that cellulose (SN, SGN, and SGKN) did not have different capabilities in inhibiting S. aureus, whereas cellulose and its composites against Candida albicans showed significantly different results (P <0.005), proving that each sample (SN, SGN, and SGKN) has the ability to inhibit Candida albicans significantly. Staphylococcus aureus is a pathogenical bacteria, having a single plasma membrane surrounded by a thick cell wall of peptidoglycan. About 90% of the cell wall is composed of peptidoglycan while the rest is a teicoic acid molecule. On the other hand, Candida albicans has a complex cell wall structure with a thickness of 100 to 400 nm. The primary composition consists of glucan, mannan, and chitin. Thus, cellulose and its composites are more susceptible to diffusion in Candida albicans because they are largely composed of chemical compounds.
The mechanism of interaction between chitosan and yeast shows that chitosan can withstand yeast growth by the destruction of biological membranes. The antimicrobial mechanism of chitosan through the interaction between the positive charge of the NH3+ group of the glucosamine unit on chitosan and the negative charge on the yeast cell membrane resulting in electrostatic interaction. A change of permeability in the yeast membrane wall can decrease internal osmotic balance to inhibit yeast growth and peptidoglycan hydrolysis of the yeast wall resulted in the loss of intracellular electrolytes, proteins, nucleic acids, and glucose in yeast.16, 24
Conclusions
Cellulose from coconut water was successfully modified by the addition of glycerol, chitosan, and silver nanoparticles. The addition of glycerol and chitosan decreased wet mass and wet yield but increased dry mass and dry yield of composites. The addition of glycerol may increase the elongation at break, but decrease the strength at break. The addition of glycerol and chitosan decreases the elongation at break and strength at break of the composite. Cellulose and cellulose composites show antibacterial activity against S.aureus, E. coli, and C. albicans. Composite of cellulose – glycerol – chitosan – silver nanoparticles shows the highest antimicrobial activity in inhibiting S. aureus at 24 hours of incubation, whereas the composite of cellulose – glycerol – silver nanoparticles shows the highest antimicrobial activity in inhibiting the growth of E. coli and C. albicans.
Acknowledgement
Thanks to Ministry of Research, Technology, and Higher Education of the Republic Indonesia for the finance support by Incentive of SINas Research 2014.
References
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Bhat, R. Int. Food Res. J. 2002, 19(1), 153-158
- Lilies, S. Peluang Usaha Nata de Coco. 2004, Kanisius, Yogyakarta, Indonesia
- Ciechańska, D.; Wietecha, J.; Kaźmierczak, D.; Kazimierczak, J. Fibres Text. East. Eur. 2010, 18(5), 98-104
- Zhang, H.; Deng, L.; Yang, M.; Min, S.; Yang, L.; Zhu, L. Int. J. Mol. Sci. 2011, 12(1), 3170-3181.
CrossRef - Torres, F. G.; Troncoso, O. P.; Torres, C.; Diaz, D.; Amaya, E. Int. J. Biol. Macromol. 2011, 48(1), 603-606 doi:10.1016/j.ijbiomac.2011.01.026.
CrossRef - Maneerung, T.; Tokura, S.; Rujiravanit, R. Carbohyd. Polym. 2007, 72(1), 1-9
- Haryono, A. and Harmami, S. B. Jurnal Kimia Indonesia, 2010, 5(1), 1-6
- Jawetz, E.; Melnick, J.; Adelberg, E. Mikrobiologi Kedokteran. 2005, Bagian Mikrobiologi FK UNAIR, edisi 1, Salemba Medika, Jakarta
- Kumar, V.; Jolivalt, C.; Pulpytel, J.; Jafari, R.; Arefi-Khonsari, F. J. Biomed. Mat. Res. A. 2012, 00A(00), 1-12
- Rosyita, D. Undergraduate Thesis. 2013, Universitas Indonesia, Jakarta, Indonesia
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Carbohyd. Polym. 2013, 94(1), 603–611.
CrossRef - Yunos, M. B. Z. and Rahman, W. A. J. Appl. Sci. 2011, 11(13), 2456-2459.
CrossRef - Li, X.; Yang, M.; Shi, X.; Chu, X.; Chen, L.; Wu, Q.; Wang, Y. Phys. E: Low-dimensional Sys. Nanostruct. 2015, 69(1), 237–242
- Tavaria, F. K.; Soares, J. C.; Reis, I. L.; Paulo, M. H.; Malcata, F. X.; Pintado, M. E. J. Appl. Microbiol. 2012, 112(5), 1034–1041.
CrossRef - Tikhonov, V. E.; Stepnova, E. A.; Babak, V. G. Carbohydrat. Polym. 2006, 64(1), 66–72.
CrossRef - Kim, K. W.; Min, B. J.; Kim, Y. T.; Kimmel, R. M.; Cooksey, K.; Park, S. I. Food Sci. Technol. 2011, 44(2), 565–569
- Singh, B. R. Medicine. 2013, 1, 1-3
- Saegeman, V. S. M.; Ectors, N. L.; Lismont, D.; Verduyckt, B.; Verhaegen, J. Burns. 2008, 34, 205-211.
CrossRef - Uznanski, P.; Zakrzewska, J.; Favier, F.; Kazmierski, S.; Bryszewska, E. J. Nanopart. Res. 2017, 19(3), 121 doi: 10.1007/s11051-017-3827-5.
CrossRef - Jin, J-C.; Xu, Z-Q; Dong, P.; Lan, J-Y.; Jiang, F-L.; Liu, Yi. 2015 doi:10.1016/j.carbon.2015.05.084.
CrossRef - Mahendra, R.; Yadav, A.; Gade, A. Biotechnol. Adv. 2009, 27, 76 – 83.
CrossRef - Handaya, A.; Joddy, A. L.; Haryono, A. Final Report. 2010, Sinergi DIKTI – LIPI, Indonesia
- Titik, I.; Siswi, S.; Harini, S. Proc. Incentive Res. 2013, KNRT, Jakarta, Indonesia
- Rechia, L.M.; Morona, J.B.J.; Zepon, K.M.; Soldi, V.; Kanis, L.A. Braz. J. Pharma. Sci. 2010, 46(3), Available at: http://dx.doi.org/10.1590/S1984-82502010000300012.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










