Kalium Hydroxide/Zirconia Pillared Bentonite for Palm Oil Transesterification
Yeslia Utubira1,2, Karna Wijaya1 , Triyono1 and Eko Sri Kunarti1
, Triyono1 and Eko Sri Kunarti1
1Department of Chemistry, Faculty of Mathematics and Natural Sciences Gadjah Mada University, Yogyakarta 55281, Indonesia.
2Department of Chemistry Education, Faculty of Teacher Training and Educational Sciences, Pattimura University, Ambon 97234, Indonesia.
Corresponding Author E-mail: karnawijaya@ugm.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340339
Article Received on : Janurary 11, 2018
Article Accepted on : May 02, 2018
Article Published : 24 May 2018
The aim of this study is to produce biodiesel from palm oil using heterogeneous catalyst i.e. KOH/ZrO2-Bentonite. The catalyst was prepared through KOH impregnation on ZrO2- Bentonite with a ratio between KOH:ZrO2-Bentonite was 20-30 wt%. Afterwards, it was followed by the heating process using microwave radiation 700 W for 10 min. Based on the result of the characterization using XRD, Gas Sorption Analyzer, FTIR and SEM-EDX, the best performance for the ratio of KOH:ZrO2-Bentonite of the process was on 25 wt%. The catalytic activity test on palm oil transesterification resulted methyl ester as much as 81% on the reaction condition of 65 oC for three hours. In addition, the amount of catalyst-used was as much as 3% of the oil weight. The result of ASTM test showed that the methyl ester product from palm oil fulfills the National Test Standard for Diesel Oil referring to SNI 04-7182-2006.
KEYWORDS:KOH; ZrO2-Bentonite; Palm oil; Transesterification
Download this article as:| Copy the following to cite this article: Utubira Y, Wijaya K, Triyono T, Kunarti E. S. Kalium Hydroxide/Zirconia Pillared Bentonite for Palm Oil Transesterification. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Utubira Y, Wijaya K, Triyono T, Kunarti E. S. Kalium Hydroxide/Zirconia Pillared Bentonite for Palm Oil Transesterification. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46174 |
Introduction
Biodiesel is an alternative energy used for diesel engine. Its characteristics are biodegradable, non-toxic, and environmental friendly. Biodiesel can be produced through transesterification of vegetable oil and animal fatty acid using alcohol and base catalyst; such as KOH, NaOH or methoxide1. The use of homogeneous catalyst for the process has been reported a lot; however, its usage has negative effects including long term regeneration time and producing a lot of toxic waste.
The use of clay as the heterogeneous catalyst has been more promising because of its positive effect including the ease of separation process between the product and the catalyst, easy to regenerate, more efficient, cheap and environmental friendly2-5. The use of heterogeneous solid catalyst from modified clay has been proven to be used in biodiesel production through transesterification process6. Base alkaline can be used as heterogeneous base catalyst if it is impregnated on supporting material. Base alkaline; such as: NaOH and KOH, is very sensitive towards free fatty acid included on oil and it is effective for transesterification process. The use of base KOH combined with supported material (alumina, NaY, MCM-4, zeolite) as heterogeneous catalyst in transesterification process is more promising. It has been used by several researchers7-10. Transesterification process of palm oil using continuous reactor, KOH/bentonite catalyst variated on KOH amount and impregnated as much as 5-25 wt%, has been successfully done. KOH impregnation of 20 wt% on bentonite has been reported that it has heterogeneous catalytic characteristics with mesoporous structure in which the biodiesel yield is higher than 75% on reaction condition of 60oC, oil/metanol mol ratio of 1:15, and rate of 0,3 mL.min-1 and active for seven days10.
The use of Indonesia natural clay as a supporting material on synthesis of heterogeneous catalyst is reached with KOH/bentonite ratio 1:4. For impregnation process, weight catalyst-used on transesterification process is as much as 3% with temperature 60oC, oil/methanol ratio 1:6, and the process takes three hours, 90,7% of biodiesel yield3. This was also found for transesterification palm oil using KOH/Al2O3 and KOH/NaY catalyst7. It can be concluded that the total amount of impregnated KOH influences on an existing of K and O activated site in catalyst. As a result, it affects biodiesel yield. The high amount of KOH will strengthen the interaction between KOH and internal bentonite layer and the calcination process produced Al-O-K group forming. This formed-group has a lower catalytic activity than alkaline K2O thus it affects the yield of biodiesel. K2O-formed due to dihydroxylation of OH groups contributes to the catalytic properties of these materials, besides the formation of K2O; as a result of the merger of ions K+ into a vacant site of zirconia with a hydroxyl group, formed K-O-Zr on the surface after the calcined process11. Several studies have reported the presence of K2O groups play an important role in improving the performance of the catalyst for the production of biodiesel10. In this study, synthesized heterogeneous base catalyst KOH/ZrO2-Bentonite was used by varying the amount of KOH is impregnated to obtain a base catalyst which has high catalytic activity for the transesterification reaction of palm oil. The catalyst activity will increase as the base sites for impregnation KOH increased.
Materials and Methods
Chemical Substances
The materials used in this study were natural Bentonite, ZrOCl2•8H2O and AgNO3 (E. Merck), KOH (E. Merck), distilled water, methanol (E.Merck) and Palm oil.
Instruments
The instruments used include: a set of glassware, centrifuge (KOKUSAN H-107), an oven (Memmert), porcelain cup, an analytical balance porcelain mortar and mortar, the 200 mesh sieve, and microwave. Diffraction patterns recorded on a XRD-6000 Shimadzu, using CuKα powder irradiated by the λ=0.5406 Å. FTIR spectra were performed on PC 8201 infrared spectrophotometer (Shimadzu) used KBr pellet at room temperature. N2 adsorption-desorption isotherms were measured at liquid nitrogen temperature with a gas sorption analyzer (Quantachrome, Autosorb-1). The morphology and elemental analysis of the test samples were estimated by Scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX ZEISS EVO MA 10).
Procedure
KOH impregnation on ZrO2-Bentonite
ZrO2-Bentonite was impregnated using KOH (20-30 wt%) solution and stirred for 24 h at room temperature. Then it was filtered and dried in an oven temperature of 70°C for 24 h. Next, it was proceed with the calcination of 700 W microwave radiation for 10 min. The impregnation results were labeled using 20%KOH/ZrO2-Bent, 25%KOH/ZrO2-Bent and 30%KOH/ZrO2-Bent. The results were characterized by XRD, Gas sorption Analyzer, FTIR, SEM-EDX.
Transesterification Palm Oil using KOH/ZrO2-Bentonite
Methanol and oil are entered into a three-neck flask (250 mL capacity) with a molar ratio of 12:1 and 3% by the weight of the catalyst KOH/ZrO2-Bentonite. The reaction was conducted at a temperature of 65oC for 3 h. Furthermore, the catalyst was separated by centrifugation. Then the mixture was put in a separating funnel and allowed to stand overnight to separate between methyl ester and glycerol. Methyl ester production was then washed with distillated hot aqua water and Na2SO4 was added into it. Methyl esters (biodiesel) were analyzed for its: density, viscosity, pour point, flash point, and heat of combustion. The analysis was determined by the method of the American Standard for Testing Materials (ASTM).
Results and Discussion
Characterization of KOH/ZrO2-Bentonite with XRD
The analysis of the KOH/ZrO2-Bentonite catalyst used XRD to determine impregnation effect of KOH towards the crystallinity of ZrO2-Bentonite. The results of X-ray diffraction analysis of KOH/ZrO2-Bentonite were shown in Fig. 1. The XRD pattern of the KOH/ZrO2-Bentonite shows the characteristics of zirconia pillared bentonite. In the total loading of KOH 20 wt%, the XRD patterns, the catalyst were still quite similar to the pillared bentonite samples and a new phase of K2O began to emerge.
The similar XRD patterns were also observed when the amount of impregnated KOH increasing to 25 wt%, crystallinity of catalyst increased. This indicates that during the process of KOH impregnation, coating/bentonite’s pillar is not physically damaged but able to absorb potassium molecules on the surface10. This is shown by the increasing intensity of the diffraction pattern of K2O observed at 2θ = 23°, 27°, 31°, 39°, 46°, 48°, 57°, and 62° (JCPDS 23-493 and JCPDS No. 019-0927)7, 12. However, the crystallinity of the catalyst decreased with increasing amount of KOH that fell to 30 wt% where the intensity of the XRD pattern declined.
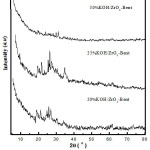 |
Figure 1: XRD pattern of KOH/ZrO2-Bentonite |
The diffractogram peak of K2O was very low compared with the prior XRD patterns of catalyst and pillared bentonite material, or there occurred an overlap with the reflection of the carrier material10. The formation of identified K2O through XRD instrumentation on the KOH/ZrO2-Bentonite showed the ununiformed dispersion of KOH dispersion either on the surface or part of pillared bentonite’s structure, which through a calcination process will turn into K2O. The K2O phase plays an important role in improving the performance of the catalyst for the production of biodiesel7, 12.
Analysis of Functional Group with FTIR
Analysis of FTIR (Fig. 2) showed the impregnation of KOH in ZrO2-pillared bentonite (ZrO2-Bentonite) afffected its material structure in which the occurrence intensity modification on some peaks of KOH/ZrO2-Bentonite. The absorption peak of wave number of 470 cm-1 decreased the intensity4, and shifted to a wave number of 462 cm-1. It indicates the formation of Si-OK bond, shown by widening band on absorption wave number of 3441 cm-1 that shifted to the wave number of 3425 cm-1.The intensity of the absorption band at wave number of 1635 cm-1 which was the vibration -OH 14, 15, increased at the spectra of 30%KOH/ZrO2-Bent because of KOH impregnation process. The shifting absorption of wave number of 1041 cm-1 on the material ZrO2-Bentonite12 to a wave number of 1033 cm-1 is predicted due to the bound metal cation of K on the cluster Si-O-H, to form Si-O-K. Based on the results of FTIR analysis, KOH impregnation of 20-30 wt% shows that the impregnation process was successful as shown by the sharpening of the absorption band at wave number of 1373 cm-1 which is the absorption of K-O and Zr-O-K bond11 that does not exist in ZrO2-Bentonite14.
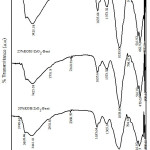 |
Figure 2: FTIR spectra of KOH/ZrO2-Bentonite |
Analysis of Surface Area and Porosity
The analysis of catalyst’s surface area used Gas Sorption Analysis (GSA), analysis of total volume of pore was determined based on the value of pore volume at P/P°~1 and the result is shown on the Table 1.
Table 1: The Analysis Result of Nitrogen Gas Adsorption
| Sample | Spesific Surface Area(m2.g-1) | Total Pore Volume(cm3.g-1) | Pore diameter, (Å) |
| 20 %KOH/ZrO2-Bent | 10.207 | 0.076 | 38.296 |
| 25 %KOH/ZrO2-Bent | 11.639 | 0.064 | 37.715 |
| 30 %KOH/ZrO2-Bent | 2.983 | 0.059 | 37.776 |
The analysis result showed that the impregnation of KOH into the ZrO2-Bentonite material decreased the specific surface area and total pore volume and increases the pore’s average diameter. The specific surface area of 20% KOH/ZrO2-Bent, 25%KOH/ZrO2-Bent and 30% KOH/ZrO2-Bent are 10.207, 11.639 and 2.983 m2 .g-1 respectively. The ratio of surface area decreases as the increasing ratio of impregnated KOH in ZrO2-Bentonite material. It indicates that KOH penetrated into the pores of ZrO2-Bentonite which causes a reduction of the surface area of the catalyst3. All catalysts show the typical type IV of adsorption isotherm type and type H1 of hysteresis loop, which is characteristic of mesoporous material with pore diameters>20 Å), as shown in Fig. 3 10,16. These results indicate that the catalyst KOH/ZrO2-Bentonite can be used as a heterogeneous base catalysts in the transesterification of palm oil.
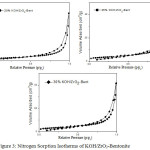 |
Figure 3: Nitrogen Sorption Isotherms of KOH/ZrO2-Bentonite |
Distribution of pore size in 25% KOH/ZrO2-Bent is more homogeneous than in the two other (Fig. 4). The addition of KOH on zirconia pillared bentonite material closed the small pores and even led to the closure of the pore-porous so it only contains the large pores.
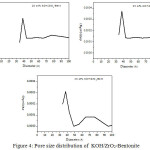 |
Figure 4: Pore size distribution of KOH/ZrO2-Bentonite Click here to View figure |
SEM – EDX Analysis
The success of impregnation process can also be observed by using SEM to investigate the morphology of the modified bentonite. Catalyst morphology of KOH/ZrO2-Bentonite using SEM (Fig. 5) showed grooves providing the distinctiveness of zirconia, pillared bentonite17. After ZrO2-Bentonite is impregnated with KOH, it shows the presence of potassium oxide granules on the surface. SEM images revealed that KOH/ZrO2-Bentonite is irregular in shape at 20000x magnification which is shown in Fig. 5 (a-c).
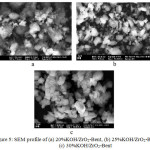 |
Figure 5: SEM profile of (a) 20%KOH/ZrO2-Bent, (b) 25% KOH/ZrO2-Bent, (c) 30%KOH/ZrO2-Bent |
After the KOH impregnation, the particles seem to form aggregates. When loading KOH 20% by weight, the surface-modified bentonite showed crystallinity which is similar to ZrO2-Bentonite. There was a reduction in the particle size of the catalyst as a result of the imposition of KOH. However, no significant differences were observed between the three catalysts. It shows a good dispersion of KOH on the surface of ZrO2-Bentonite. For loading KOH 25 wt%, the morphology of the catalyst has greater pores between the particles of the 20%KOH/ZrO2-Bent and 30%KOH/ZrO2-Bent. The more homogeneous it is (Fig. 5a, 5c) it leads to increased contact between the catalyst and the substrate, there by increasing the catalytic ability in the transesterification reaction18. The morphology of 30%KOH/ZrO2-Bent changed to an amorphous structure because the structure of the pillared bentonite was broken. It is supported by the data crystallinity on XRD. Excess potassium at 30 wt% covers all pores and pillared bentonite surface, thus it affects to the growth of the particles. The existence of potassium oxide impregnated in ZrO2-Bentonite was reinforced by using EDX. The characterization result of the catalyst with the imposition of KOH (20-30 wt%) are respectively 12.93%, 17.69% and 6:04%. It is presented in Table 2.
Table 2: Potassium Content of prepared catalyst from EDX analysis
| Catalyst | K (wt%) |
| 20%KOH/ZrO2-Bent | 12.93 |
| 25%KOH/ZrO2-Bent | 17.69 |
| 30%KOH/ZrO2-Bent | 6.40 |
Palm Oil Transesterification
The activities of KOH/ZrO2-Bentonite as heterogeneous base catalysts which have different base strength were tested in the transesterification reaction of palm oil.
Table 3: Transesterification result of Palm oil using KOH/ZrO2-Bentonite
| Catalyst | MeOH//oil molar ratio | Amount of catalyst (%) | Reaction Time (h) | Conversion of biodiesel (%) |
| 20%KOH/ZrO2-Bent | 15:112:1 | 33 | 33 | 55.074.65 |
| 25%KOH/ZrO2-Bent | 9:115:112:112:112:1 | 33333 | 33123 | 40.064.050.060.081.0 |
| 30%KOH/ZrO2-Bent | 15:112:1 | 33 | 23 | 61.572.0 |
* SNI 04-7182-2006
Transesterification was performed under the conditions which was equal to the weight of the catalyst-used 3% of heavy oil. Based on the data in Table 3, the highest conversion of biodiesel product using a catalyst of 25%KOH/ZrO2-Bent is 81% with a mole ratio methanol:oil 12:1 and temperature of 65 °C which is proceed for 3 h. Table 3 reveals the number of loading KOH from 20-30 wt% which increases the yield of the highest biodiesel (81%) obtained for the loading of KOH 25 wt% on pillared bentonite material. However, when the amount of KOH increased as much as 30 wt%; biodiesel product decreased. This is due to the agglomeration occurs because the excess of KOH alkaline led to a decrease in surface area of the catalyst, so that its activity is reduced. Besides, loading amount of KOH is high (30 wt%) caused the formation of a new phase (Al-OK) which has properties alkalinity and catalytic activity is lower than the phase K2O 4, 7, 9. Mole ratio of methanol:oil also affects to biodiesel product where in the mole ratio of methanol: oil 15:1 affects to declining on the conversion of biodiesel products due to the separation of the glycerol becomes more difficult7. GC-MS analysis was carried out on the conversion of palm oil biodiesel products of the highest (81%) to identify the components methyl ester compound contained qualitatively presented in Fig. 6.
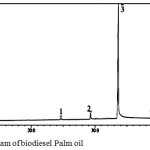 |
Figure 6: Chromatogram of biodiesel Palm oil |
The result of GC analysis shows 6 peaks were detected as methyl ester which indicates the existences of 6 methyl ester. Each GC peak is analyzed by MS, it is derived methyl esters which is contained in biodiesel products of palm oil including: methyl laurate (0.70%), methyl myristate (1.99%), methyl palmitate (49.78%), methyl stearate (2.76%), methyl oleic (35.41%) and methyl linoleic (9.35%)
Biodiesel Characterization
Characteristic results of biodiesel from palm oil are shown in Table 4.
Table 4: The properties of biodiesel produced in this experiment
| Parameter | Diesel oil *) | ASTM | Palm oil biodiesel |
| Specific density 60/60oF | 0,85-0,92 | 870-900 at 15oC | 0,8771 |
| Kinetic viscosity on 40oC, mm2. s-1 | 2,3-6,0 | 1.9 – 6.0 | 5.730 |
| Pour point, oC | Maks. 18 | 15 to 10 | 9 |
| Flash point, oC | Min. 100 | Min. 130 | 165 |
| Cloud point, oC | Max. 18 | 3 to 12 | 15 |
| Water content, %vol | Max. 0,05 | Max. 0.03 | trace |
The viscosity of biodiesel fuel is 5.730 mm2.s-1. It gives a significant influence on the performance of injector and atomization of the fuel. Likewise, the density of biodiesel is as desired. The pour point and cloud point of biodiesel also meet the standards as a fuel. High pour point made a difficulty of ignited engine at low temperatures. The Flash point is related to the evaporation of the fuel. It is because flash point of palm oil biodiesel is higher than what is written in ISO 2006, consequently making it safe for storage purpose for a long time. Therefore, it can be used as substitution fuel for diesel engines.
Conclusion
KOH/ZrO2-Bentonite from synthesis product can be used as a heterogeneous base catalyst in the transesterification reaction of palm oil. The results of transesterification of palm oil using the catalyst KOH/ZrO2-Bentonite produced biodiesel with the optimum conversion of 81% was under the reaction conditions of 65oC, molar ratio methanol: oil 12 : 1, and the weight of the catalyst used at 3% for 25%KOH/ZrO2-Bent.
Acknowledgement
The financial support from DRPM Kemenristek Dikti under Hibah Disertasi Doktor scheme research granted in 2017 is gratefully acknowledged.
References
- Alves, H. J.; da Rocha, A.M.; Monteiro, M.R.; Moretti, C.; Cabrelon, M.D.; Sclwengher, C.A.; and Milinsk, M.C. Appl. Clay Sci., 2014, 91–92, 98–104.
CrossRef - Hasanudin, M; Said, M; Faizal, M. H. D.; Wijaya, K. Sustainable Environ. Res. 2012, 22, 395-400.
- Soetaredjo, F. E.; Ayucitra, A.; Ismadji, S.; Maukar, A. L. Appl. Clay Sci. 2011, 53, 341–346.
CrossRef - Wijaya, K.; Iqmal, T; Ahmad, B., Indones. J. Chem. 2002, 2, 12-21.
- Nagendrappa, G., Resonanc. 2002, 7, 64-77.
- de Oliveira, A. D. N.; da Silva Costa, L. R.; de Oliveira Pires, L. H.; do Nascimento, L.A.S.; Angélica, R. S.; da Costa, C. E. F.; Zamian, J. R.; da Rocha Filho, G. N. Fuel. 2013, 103, 626-631.
CrossRef - Noiroj, K.; Intarapong, P.; Luengnaruemitchai, A.; Jai-In, S. J. Renewable Energy. 2009, 34, 1145–1150.
CrossRef - Rashtizadeh, E.; Farzaneh, F.; Ghandi, M. Fuel. 2010, 89, 3393–3398.
CrossRef - Agarwal, M.; Chauhan, G.; Chaurasia, S. P.; Singh, K., J. Taiwan. Inst. Chem. Eng. 2012, 43, 89–94.
CrossRef - Intarapong, P.; Jindavat, C.; Luengnaruemitchai, A.; Samai, J-I., Int. J. Green Energy. 2014, 11, 987–1001.
CrossRef - Takase, M.; Zhang, M.; Feng, W.; Chen, Y., Zhao, T.; Cobbina, S. J.; Liuqing, Y.; Wu, X., Energy Convers. Manage. 2014, 80, 117–125.
CrossRef - Xie, W.; Lie, H. J. Mol. Catal. A. Chem. 2006, 255, 1–9.
CrossRef - Suseno, A.; Wijaya, K.; Trisnunaryanti, W.; Shidiq, M. Asian. J. Chem. 2015, 27, 2619-2623.
CrossRef - Utubira, Y.; Wijaya, K.; Triyono; Kunarti, E. S. Int. J. ChemTech. Res. 2016, 9, 475-482.
- Sekewael, S.J.; Wijaya, K.; Triyono; Budiman, A. Asian. J. Chem. 2016, 28, 2325-2330.
CrossRef - Singh, V.;Sapehiyia, V.; Kad, G. L., J. Mol. Catal. A: Chem. 2004, 210, 119–124.
CrossRef - Fatimah, I.; Rubiyanto, D.; Huda, T., Int. J. Chem. Eng., 2014.
- Qiu, F.; Li, Y, Yang, D.; Li, X.; Sun, P., Appl. Energy. 2011, 288, 2050–2055.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









