Investigating Oxidation of Formaldehyde over Co3o4 Nanocatalysts at Moderate Temperature
Manpreet Singh and Kamal Kishore
and Kamal Kishore
Department of Chemistry, Akal College of Basic Sciences, Eternal University, Baru Sahib, Sirmour-173101, H.P, India.
Corresponding Author E-mail: manpreetrisam19@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340327
Article Received on : April 19, 2018
Article Accepted on : May 17, 2018
Article Published : 14 Jun 2018
Formaldehyde is considered as one of the major contributor to global air pollution having extreme toxicity even at very low concentrations in air. Evidently, efficient processes and effective materials for the abatement of formaldehyde at low temperature are of great significance. In this paper, we report the comparative study for the catalytic oxidation of formaldehyde in aqueous medium at moderate temperature conditions (20-40oC). The catalysts used are nanoparticles of Co3O4 synthesized via chemical combustion, sol-gel autocombustion, thermal decomposition and hydrothermal route with particle size 21 ± 1 nm, 39 ± 1 nm, 16 ± 1 nm and 25 ± 1 nm respectively. The characterization of the synthesized nanocatalysts was performed by X-ray diffractometry (XRD), Transmission electron microscopy (TEM), Fourier-transform infrared (FT-IR) spectrum. The XRD measurements confirm the cubic spinel phase of Co3O4 nanoparticles. Mechanism for the oxidation of formaldehyde has also been proposed.
KEYWORDS:Cobalt Oxide; Catalytic Oxidation; Formaldehyde; Nanoparticles
Download this article as:| Copy the following to cite this article: Singh M, Kishore K. Investigating Oxidation of Formaldehyde over Co3o4 Nanocatalysts at Moderate Temperature. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Singh M, Kishore K. Investigating Oxidation of Formaldehyde over Co3o4 Nanocatalysts at Moderate Temperature. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46640 |
Introduction
Volatile organic compounds (VOCs) with boiling points ranging between room temperature and 260oC are considered as major contributors to global air pollution.1 They are the main contributors to photochemical smog, secondary aerosol and many VOCs (such as benzene and toluene) are found to be harmful to living beings due to their toxic, malodorous, mutagenic and carcinogenic nature.2,3 Formaldehyde emitted widely from building and decorative materials is becoming a major pollutant and it also has photochemical activity in atmosphere.4
Formaldehyde is considered harmful to human health if it exceeds safe limitations. Serious health problems such as burning sensation in the eyes, irritation in throat, difficulty in breathing and even pernicious diseases such as nasopharyngeal or nasal cancer can be caused because of excessive exposure to a formaldehyde polluted environment.5 Due to its effects on human health and environment, its emission reduction is of significant interest particularly at room temperature.
Several methods have been used to control formaldehyde oxidation6,7 but catalytic oxidation is considered as one of the best alternative as it converts formaldehyde into non harmful carbon dioxide and water. Therefore, it is desirable to synthesize catalysts that can oxidize formaldehyde. It has been found that transition metal oxides (Fe2O3, MnO2, NiO and Co3O4) and rare earth metals or composite oxides (CeO2, LaMnO3 and LaFeO3) have been used to catalytically oxidize formaldehyde. However, these catalysts operate at high temperatures i.e. 200-300oC and their catalytic efficiency is inadequate for technological adaptation.8-10 Au based catalysts have shown high activity towards catalytic oxidation of formaldehyde at moderate temperatures11-14 but their high cost limit their use. Thus, the catalytic oxidation of formaldehyde using low cost catalysts at low/moderate temperatures is still a desirable goal.
Nano-structures of inorganic materials can lead to novel magnetic, optical, electronic and catalytic properties as compared to their bulk counterparts.15-17 It has been assumed that these materials may possess numerous edges and corners for adsorption and activation of reaction or possess several kinds of active sites, which may exhibit different reactivities and typically higher activity.
Nano Co3O4 with morphologies such as tube, sheet, belt, rod, sphere and pore has shown excellent catalytic performance and is used in various fields.18-22 Morphology and structure of Co3O4 has an important role in the catalytic performance of the catalyst.23-25 Co3O4 is a p-type semiconductor and its spinel structure is based upon cubic close packing array of oxide ions in which Co (II) ions occupy the tetrahedral 8a sites and Co (III) ions occupy the octahedral 16d sites.26 Out of different crystal faces of Co3O4, the naturally exposed surface of Co3O4 is the (110) crystal face and it plays an important role in the catalytic oxidation.27 It has also been observed that cobalt oxide readily oxidizes carbon monoxide to CO2 by a dynamic oxygen transfer from the air.28 In this paper, nano cobalt oxides synthesized via, chemical combustion, sol-gel autocombustion, thermal decomposition and hydrothermal method are comparatively studied for the catalytic oxidation of formaldehyde under mild/moderate temperature conditions (20-40oC) in aqueous medium. A general mechanism for the oxidation of formaldehyde on the surface of nanocatalyst has also been proposed.
Experimental
Chemicals were purchased from Merck, India. All the chemicals were of analytical grade and used without further purification. Double distilled water was used in all the experiments.
Chemical Combustion
Cobalt nitrate (0.1 mol; 29.103 g) and ethylene glycol/polyethylene glycol (EG/PEG) in stoichiometric proportions were dissolved and stirred followed by the addition of urea (0.1 mol, 6.0g). The temperature was raised up to 70oC and stirring was continued until combustion occurred. The powder was crushed, grounded and then washed with water and methanol to remove any unwanted impurities. The product obtained was then dried and further annealed at 600oC in ceramic crucible in a programmed furnace so as to obtain a pure crystalline phase. The crystalline powder obtained is designated as Co3O4-CC.
Sol-gel Autocombustion
Stoichiometric amount of cobalt chloride (0.1 mol) and citric acid were dissolved in ethanol:water (1:5) solution and was continuously stirred. The pH of the solution was adjusted to 7 using ammonia. The solution was then heated and stirred continuously to transform it into xerogel. At a proper temperature, ignition started and the dried gel burnt it in a self-propagating combustion manner until whole the gel was burnt out completely to form a loose powder. The desired powder was calcined at 450oC for three hours. The crystalline powder obtained is designated as Co3O4-SG.
Thermal Decomposition Method
The material and method used to prepare nano cobalt oxide via thermal decomposition method has already been published.29 The crystalline powder obtained is designated as Co3O4-TD.
Hydrothermal Method
CoCl2.6H2O (0.05 mol) was dissolved in 40 ml distilled water followed by stirring for about one hour. KOH was added as precipitating agent to increase the pH up to 13 followed by stirring for half an hour to prepare a homogenous sol. Whole material was then transferred to a teflon vessel of capacity 100 ml and was kept in a stainless steel autoclave which in turn was put in a programmed furnace at 180oC for 48 hours. The precipitates so obtained were washed with water and ethanol to remove the suspended impurities and were subjected to heating for 8 regular hours at 80oC in a programmed oven. The crystalline powder obtained is designated as Co3O4-HT.
Characterization
The spectrum of Co3O4 nanoparticles were studied in the infrared region by Fourier transform infrared spectroscopy (FTIR) using a Nicolet Avtar 5700 system. The structure of the catalyst was studied by X-ray diffraction (XRD) using X-Pert PROsystem. The morphology of the catalyst was studied by Transmission electron microscopy (TEM) using Hitachi H-7500.
Catalytic Activity
Oxidation of Formaldehyde
10 ml HCHO (1%), 20 ml K2Cr2O7 (0.1 M) solution and 100 ml dil. H2SO4 were taken in a closed lid 250 ml conical flask. To this flask, 100 mg of the synthesized metal oxide nano catalysts were added. The solutions were ultrasonicated for 5 minutes at three different temperatures i.e. 20, 30 and 40oC followed by filtration using sintered glass crucibles and the filtrate was titrated against 0.1 M mohr salt solution using diphenyl amine as an indicator. Appearance of green color gave the end point. The comparison was done with the blank observation i.e. without the catalyst. The readings obtained for the titration were recorded and are shown in Table 1 and Figure 4 respectively.
Results and Discussion
The FTIR spectrum of the Co3O4 nanostructures synthesized via chemical combustion (Co3O4-CC), sol-gel autocombustion (Co3O4-SG), thermal dcomposition (Co3O4-TD) and hydrothermal method (Co3O4-HT) are shown in Figure 1.
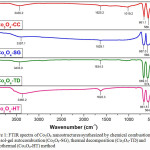 |
Figure 1: FTIR spectra of Co3O4 nanostructures synthesized by chemical combustion (Co3O4-CC), sol-gel autocombustion (Co3O4-SG), thermal decomposition (Co3O4-TD) and hydrothermal (Co3O4-HT) method |
The figure clearly represents the metal-oxygen absorption bands, characteristic of cubic spinel structure of Co3O4. The absorption peaks at 568.1 cm-1 and 661.1 cm-1 (Co3O4-CC), 568.0 cm-1 and 667.0 cm-1 (Co3O4-SG), 574.3 cm-1 and 669.3 cm-1 (Co3O4-TD) and 564.4 cm-1, 661.5 cm-1 (Co3O4-HT) represents stretching vibrations of metal–oxygen (Co–O) bonds and confirm the formation of the Co3O4 spinel oxide. The minor peak at 1019.27 cm-1 observed in Co3O4-CC sample may be attributed to C-H stretching vibrations from EG/PEG.30 The broad bands near 3430.2 cm-1 (Co3O4-CC), 3397.1 cm-1 (Co3O4-SG), 3433.0 cm-1 (Co3O4-TD) and 3365.2 cm–1 (Co3O4-HT) correspond to stretching vibrations of hydrated water. The less intensive bands at 1625.2 cm-1 (Co3O4-CC), 1629.1 cm-1 (Co3O4-SG), 1634.3 cm-1 (Co3O4-TD) and 1622.3 cm–1 (Co3O4-HT) are attributed to bending vibration of water molecules.31
The XRD pattern of Co3O4 nanostructures synthesized via chemical combustion (Co3O4-CC), sol-gel autocombustion (Co3O4-SG), thermal decomposition (Co3O4-TD) and hydrothermal method (Co3O4-HT) are shown in Figure 2. XRD graphs indicate the formation of polycrystalline spinel phase. All diffraction peaks in this pattern could be indexed to a cubic spinel Co3O4 with lattice parameter a = 8.083 Ao (JCPDS No. 42-1467) and correspond to (111), (220), (311), (222), (400), (422), (511), and (440) reflections of cubic spinel Co3O4 with Fd3m [227] space group.
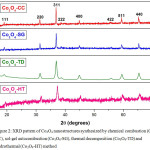 |
Figure 2: XRD pattern of Co3O4 nanostructures synthesized by chemical combustion (Co3O4-CC), sol-gel autocombustion (Co3O4-SG), thermal decomposition (Co3O4-TD) and hydrothermal (Co3O4-HT) method Click here to View figure |
The average crystallite sizes for the most intense peak [(311) plane] were calculated from the XRD data using Scherrer formula and were found to be 21 nm, 39 nm, 16 ± 1 nm and 24 nm for Co3O4-CC, Co3O4-SG, Co3O4-TD and Co3O4-HT respectively. The X-ray density of prepared specimens was calculated using equation 1:
![]()
Where Z is the number of molecules per unit cell, M the molecular weight, a the lattice parameter and N the Avogadro’s number. Sharp peaks in the pattern suggest that the sample has good crystallinity indicating the formation of pure Co3O4 spinel phase.
The TEM images of Co3O4 nanostructures synthesized via chemical combustion (Co3O4-CC), sol-gel autocombustion (Co3O4-SG), thermal decomposition (Co3O4-TD) and hydrothermal method (Co3O4-HT) are shown in Figure 3.
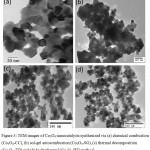 |
Figure 3: TEM images of Co3O4 nanocatalysts synthesized via (a) chemical combustion (Co3O4-CC), (b) sol-gel autocombustion (Co3O4-SG), (c) thermal decomposition (Co3O4-TD) and (d) hydrothermal (Co3O4-HT) method Click here to View figure |
The observed micrograph shows homogeneity and uniformity. The high-resolution TEM images shows that the Co3O4 nanoparticles are having almost cubical shape with mean crystallite size 21 nm, 38 ± 1 nm, 16 ± 1 nm and 25 ± 1 nm for Co3O4-CC, Co3O4-SG, Co3O4-TD and Co3O4-HT respectively. There is consistency between the values observed by TEM and XRD.
A series of experiment were designed in order to study the comparative catalytic oxidation of formaldehyde by Co3O4 synthesized via chemical combustion (Co3O4-CC), sol-gel autocombustion (Co3O4-SG), thermal decomposition (Co3O4-TD) and hydrothermal method (Co3O4-HT). To study the reaction in aqueous medium and at moderate temperature conditions (20-40oC) was the area of interest. The results of the experiment are shown in Table 1 and Figure 4.
Table 1: Titration results for the oxidation of formaldehyde by Co3O4 nanocatalysts synthesized via chemical combustion (Co3O4-CC), sol-gel autocombustion (Co3O4-SG), thermal decomposition (Co3O4-TD) and hydrothermal (Co3O4-HT) method
|
S. No. |
Catalyst |
20oC (in ml) |
30oC (in ml) |
40oC (in ml) |
|
1 |
Without Catalyst |
15.0 |
13 |
9.8 |
|
2 |
Co3O4-CC |
10.4 |
8.8 |
7.1 |
|
3 |
Co3O4-SG |
7.3 |
6.6 |
5.4 |
|
4 |
Co3O4-TD |
6.7 |
5.8 |
4.2 |
|
5 |
Co3O4-HT |
7.7 |
6.1 |
4.0 |
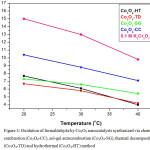 |
Figure 4: Oxidation of formaldehyde by Co3O4 nanocatalysts synthesized via chemical combustion (Co3O4-CC), sol-gel autocombustion (Co3O4-SG), thermal decomposition (Co3O4-TD) and hydrothermal (Co3O4-HT) method |
Figure 4 represents the oxidation of 1% HCHO using all synthesized nanocatalysts at 20, 30 and 40oC where Co3O4-CC, Co3O4-SG, Co3O4-TD and Co3O4-HT represents Co3O4 synthesized by chemical combustion, sol-gel autocombustion, thermal decomposition and hydrothermal method respectively. The graph represents that all the synthesized catalysts have the capacity to oxidize formaldehyde. This may be attributed to the fact that nanomaterials increase the exposed surface area of the active component of the catalyst thereby enhancing the contact between reactants and catalyst. The oxidation by each catalyst is compared with the blank reading of 0.1 M K2Cr2O7.
It is found that with the increase in temperature, rate of reaction increases i.e as the temperature increases, the catalytic activity of the catalyst increases. Thus, under similar conditions of concentration and temperature, the extent of oxidation increases in the order 20oC<30oC<40oC. However, the catalytic performance is markedly influenced by the methods employed for the synthesis of nanocatalysts. It is observed that catalyst annealed at high temperatures (Co3O4-CC) have low activity as compared to the catalysts annealed at comparatively lower temperatures (Co3O4-SG, Co3O4-TD and Co3O4-HT). This may be due to the fact that at high temperatures, sintering of the catalyst occurs which makes the material less porous by deactivating the active sites. However, catalysts annealed at low temperatures retain their porous nature and hence activity. The study of the oxidation of formaldehyde establishes the concept of green chemistry as it is studied at mild temperature conditions and in aqueous medium. Being heterogeneous, the synthesized catalysts are easy to remove and reuse. The nanosize of the catalyst imparts high surface area for better contact. Thus the study reveals that the nano oxides of Cobalt are capable of oxidizing organic molecules as catalyst at mild temperature conditions. Figure 4 clearly shows that out of all the catalysts synthesized, Co3O4-TD and Co3O4-HT have shown exceptionally high catalytic activity. Much literature is not available in this respect.
A general mechanism for the oxidation of formaldehyde on the surface of nano CO3O4 has also been proposed. Firstly, formaldehyde will adsorb on the surface of the nanocatalyst. The formaldehyde molecules orientate themselves on the nanosized cobalt oxide (Co3O4) catalyst having a surface with alternate Co2+ and Co3+ sites by making vander waals interactions between O of carbonyl (CO) and Co3+ whereas the C of methylene (-CH2) remains free. This facilitates the attack of oxygen (O2) on the C of methylene (-CH2) and leading to its conversion in to CO2 and H2O as shown in figure 5.
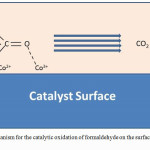 |
Figure 5: Mechanism for the catalytic oxidation of formaldehyde on the surface of Co3O4 nano catalyst Click here to View figure |
Thus, the process is a continuous adsorption of formaldehyde and oxygen on the active surfaces of Co3O4 consisting of Co2+ and Co3+ sites and degradation of toxic formaldehyde to carbon dioxide and water molecules.
Conclusion
Low cost nano transition metal oxide of Co3O4 were synthesized via chemical combustion, sol-gel autocombustion, thermal decomposition and hydrothermal route; and were comparatively studied as catalyst for the oxidation of formaldehyde in aqueous medium and at moderate temperature conditions (20-40oC). These Co3O4 nanoparticles synthesized via thermal decomposition and hydrothermal route have shown good catalytic activity towards the oxidation of formaldehyde and hence they are found as useful catalysts for dynamic oxygen transfer reactions.
Acknowledgement
The author would like to thank SAIF-Panjab University, Chandigarh, USIC-Himachal Pradesh University, Shimla and Tinchem Enterprises (www.tinchementerprises.in) for their liberal support.
References
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D. Y. C.; Catal. Sci. Technol. 2015, 5, 2649-2669.
CrossRef - Amann, M.; Lutz, M.; J. Hazard. Mater. 2000, 78, 41-62.
CrossRef - Li, N.; Gaillard, F. Appl. Catal., B. 2009, 88, 152-159.
CrossRef - Bai, B.; Arandiyan, H.; Li, J. Appl. Catal., B. 2013, 142-143, 677-68.
CrossRef - Hu, L.; Peng, Q.; Li, Y. J. Am. Chem. Soc. 2008, 130, 16136-16137.
CrossRef - Zhang, J.; Jin, Y.; Li, C.; Shen, Y.; Han, L.; Hu, Z.; Di, X.; Liu, Z. Appl. Catal., B. 2009, 91, 11-20.
CrossRef - Chen, X.; Zhu, H.; Zhao, J.; Zheng, Z.; Gao, X. Angew. Chem. 2001,120, 5433-5436.
CrossRef - Zheng, Z.; Teo, J.; Chen, X.; Liu, H.; Yuan, Y.; Waclawik, E.; Zhong, Z.; Zhu, H. Chem.-Eur. J. 2010, 16, 1202-1211.
CrossRef - Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Loannides, T.; Verykios, X. J. Catal. 1998, 178, 214-225.
CrossRef - Spinici, R.; Faticanti, M.; Marini, S.; De, R. S.; Porta, P. J. Mol. Catal. A: Chem. 2003, 197, 147-155.
CrossRef - Ma, C.; Wang, D.; Xue, w.; Dou, B.; Wang, H.; Hao, Z. Environ. Sci. Technol. 2011, 45, 3628-3634.
CrossRef - Shen, Y.; Yang, X.; Wang, Y.; Zhang, Y.; Zhu, H.; Gao, L.; Jia, M. Appl. Catal., B. 2008,79, 142-148.
CrossRef - Li, C.; She, Y.; Jia, M.; Sheng, S.; Adebajo, M. O.; Zhu, H. Catal. Commun. 2008, 9, 355-361.
CrossRef - Wang, Y.; Zhao, D.; Ma, W.; Chen, C.; Zhao. J. Environ. Sci. Technol. 2008, 42, 6173-6178.
CrossRef - Hrdlicka, J. A.; Seames, W. S.; Mann, M. D.; Muggli, D.S.; Horabik, C. A. Environ. Sci. Technol. 2008, 42, 6677-6682.
CrossRef - Hupp, J. T.; Poeppelmeier, K. R. Science. 2005, 309, 2008-2009.
CrossRef - Huang, M. H.; Mao, S.; Feick, H.; Yan, H.; Wu,Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Science. 2001, 292, 1897-1899.
CrossRef - Chen, J.; Xu, L. N.; Li, W. Y.; Gou, X. L. Adv. Mater. 2005, 17, 582-586.
CrossRef - Meher, S. K.; Rao, G. R. J. Phys. Chem.C. 2011, 115, 15646-15654.
CrossRef - Yamada, Y.; Yano, K.; Xu, Q.; Fukuzumi, S. J. Phys. Chem.C. 2010, 114 16456-16462.
CrossRef - Hilgendorff, M.; Tesche, B.; Giersig, M. Aust. J. Chem. 2001, 54, 497-501.
CrossRef - Yang, J.; Sasaki, T.; Cryst. Growth Des. 2010, 10, 1233-1236.
CrossRef - Ma, C.; Mu, Z.; Li, J.; Jin, Y.; Cheng, J.; Lu, G.; Hao, Z.; Qiao, S. Am. Chem. Soc. 2010, 132, 2608-2613.
CrossRef - Xie, X.; Li, Y.; Liu, Z.; Haruta, M.; Shen, W. J. Nature. 2009, 458, 746-749.
CrossRef - Ma, C.; Wang, D.; Xue, W.; Dou, B.; Wang, H.; Hao, Z. Environ. Sci. Technol . 2011, 45, 3628-3634.
CrossRef - Jing, X.; Song, S.; Wang, J.; Ge, L.; Jamil, S.; Liu, Q.; Mann, T.; He, Y.; Zhang, M.; Wei, H.; Liu, L. Powder Technol. 2012, 217, 624-628.
CrossRef - Xu, X. L.; Li, J. Q. Surf. Sci. 2011, 605, 1962-1967.
CrossRef - Lin, H. K.; Chiu, H. C.; Tsai, H. C.; Chien, S. H.; Wang, C. B. Catal. Lett. 2003, 88, 169-174.
CrossRef - Singh, M.; Ralhan, N. K.; Singh, S. Bull. Mater. Sci. 2015, 38, 297-301.
CrossRef - Yan, Q.; Li, X.; Zhao, Q.; Chen, G. J. Hazard. Mater. 2012, 209, 385-391.
CrossRef - Alizadeh-Gheshlaghi, E.; Shaabani, B.; Khodayari, A.; Azizian-Kalandaragh, Y.; Rahimi, R. Powder Technol. 2012, 217, 330-339.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









