Computational Evaluation of Novel Schiff Base Complexes as Anti Dengue and Anti-Cancer Agent
M. Seethalakshmi and T. Peter Amaladhas
Research Department of Chemistry, V.O. Chidambaram College, Tuticorin,Affiliated to Manonmaniam Sundaranar University, Tirunelveli.
Corresponding Author E-Mail: peteramaladhas@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340330
Article Received on : January 08, 2018
Article Accepted on : May 11, 2018
Article Published : 29 May 2018
A neoteric Schiff base ligand, 4-(4-chlorobenzylideneamino)-N-(4-(4-chlorobenzylideneamino) phenyl)benzamide (L1), and its Co(II), Ni(II) and Cu(II) complexes were synthesized. The structure of ligand L1 and its complexes were confirmed by NMR, FT-IR, UV-Vis., EPR and EI- mass techniques. The synthesized complexes were docked with human DNA to poisomerase I (PDB: 1SC7) and Dengue NS3 protease-helicase (PDB ID: 2VBC) using Auto Dock vina and Discovery studio software. The binding which is enclosed in a box having number of grid points in x × y × z directions of 40 × 40 × 40 and a grid spacing of 0.35 Å. The binding energy values of the Co(II), Ni(II) and Cu(II) complexes were respectively -12.4, -13.7 and -13.0 kcal mol−1 towards NS3 protease-helicase and -16.8, -10.4 and -15.1 kcal mol−1 towards human DNA topoisomerase I. These results established that the synthesized compounds could act as a potential Anti-Dengue and Anticancer agent.
KEYWORDS:Anti-Dengue Drug; Docking; Human DNA Topoisomerase I; NS3 Protease-Helicase; Schiff Base Ligand
Download this article as:| Copy the following to cite this article: Seethalakshmi M, Amaladhas T. P. Computational Evaluation of Novel Schiff Base Complexes as Anti Dengue and Anti-Cancer Agent. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Seethalakshmi M, Amaladhas T. P. Computational Evaluation of Novel Schiff Base Complexes as Anti Dengue and Anti-Cancer Agent. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46249 |
Introduction
Research on interactions of the metal complexes especially Co(II), Ni(II) and Cu(II) with DNA helps to get a immense knowledge about development of novel chemotherapeutics and medicines [1-4]. The interactions of metal complexes with DNA usually cause damage in cancer cells [5-7]. Dengue virus basically belongs to Flavivirus genus and is a member of Flaviviridae family [8,9]. The major source of the dengue virus development is human and monkey. Usually dengue affected humans have severe flu, fever, body pain like symptoms and body temperature almost reaches 40°C which makes human to be critical. Severe headache, flushing of face and rashes in skin are the extreme symptoms of Dengue fever and this severe condition is said to be Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS) [10,11]. It is not possible to do the all experiment on the interaction of drugs towards the targeting molecule in low cost. Docking is the only way to give the corresponding exact results of newly synthesized drugs towards the targeting molecule [12-18]. Auto dock vina and discovery studio is a useful tool to study the interaction between the ligand and the target molecule. This software helps to determine the binding mode, binding affinity and also the exact stable configuration of the ligand, which binds with the receptor [19-22]. Using this docking study, we can understand the coordination mode and the binding nature. It will help to design a good drug for the disease [23-26]. In this present work, a neoteric Schiff base ligand, 4-(4-chlorobenzylideneamino)-N-(4-(4-chlorobenzylideneamino) phenyl)benzamide (L1) and Co(II), Ni(II) and Cu(II) complexes of L1 were synthesized. The synthesized ligand and its complexes were treated with human DNA topoisomerase I (PDB: 1SC7) and Dengue NS3 protease-helicase (PDB ID: 2VBC) by docking.
Materials and Experimental Methods
All the chemicals which were used in the present work were purchased from commercial sources and were used without further purification. 4,4′-diaminobenzanilide, p-chlorobenzaldehyde, cobalt chloride hexahydrate (CoCl2. 6H2O), nickel chloride hexahydrate (NiCl2. 6H2O) and copper chloride dihydrate (CuCl2. 6H2O) were bought from Sigma Aldrich, USA and utilized as received. Solvents used in the present research were purchased from Merck and were used without further purification.
1H-NMR spectrum of the synthesized Schiff base ligand L1 was recorded in DMSO (d6) and TMS is used as an internal standard on a Bruker Advance DRX 300 FT-NMR instrument. Vibrational spectra of the ligand and complexes were recorded in JASCO/FT-IR410 spectrometer in the range of 4000-400 cm-1 at 16 scans/min. Potassium bromide disc method was employed for sample preparation. Electronic spectra of the complexes dissolved in DMSO were recorded using Perkin Elmer Lambda-25 UV-Vis. spectrophotometer in the range of 200-800 nm. The Electron Ionization Mass spectra of the ligand was recorded using JEOL DX-303 EI mass spectrometer, and X- band EPR spectra of the copper complex in DMSO was recorded on Varian E-4 X-band spectrometer using DPPH as the g-marker at room temperature at Indian Institute of Technology, Madras, India.
Experimental
Synthesis of 4-(4-chlorobenzylideneamino)-N-(4-(4-chlorobenzylideneamino)phenyl) benzamide (L1)
A hot solution of 1.136 g (5 mmol) 4,4′-diaminobenzanilide in 10 mL methanol was added drop wise to a hot stirring solution of 1.4057g (10 mmol) p-chlorobenzaldehyde in10 mL methanol. The above mixture was stirred under reflux condition for 5 hours (Scheme-1). A yellow colour solid mass appeared was filtered, washed with dimethyl ether several times and dried in in vacuo at room temperature.
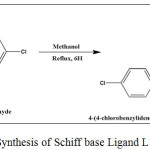 |
Scheme 1: Synthesis of Schiff base Ligand L1 |
Synthesis of Cobalt(II), Nickel(II) and Copper (II) complexes of L1
To the hot stirring solution of the 0.9447 g (2 mmol) Schiff base ligand L1 in 20 mL of methanol, the corresponding metal(II) chlorides [CoCl2.6H2O (0.237 g, 1 mmol), NiCl2.6H2O (0.2379 g, 1 mmol) and CuCl2.2H2O (0.170 g, 1 mmol)] in 20 mL of methanol were added, stirred under reflux for 6 hours (Scheme-2). Then the product obtained was filtered, washed and dried in vacuo at room temperature.
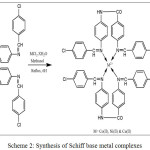 |
Scheme 2: Synthesis of Schiff base metal complexes
|
Molecular Docking Study
Human‐DNA–Topo‐I complex (PDBID: 1SC7) [27] and Dengue NS3 protease-helicase (PDB ID: 2VBC) [28] were obtained from Protein Data Bank (http://www.rcsb.org/pdb). Metal complexes were converted into PDB format by using Mercury software [29]. The ‘receptor’ (DNA/Dengue) and ‘ligand’ (metal complexes) files were docked using AutoDock Tools. The hetero atoms such as water molecules and excess ligand were removed. The polar hydrogen atoms and Kollman charges were added to the receptor (DNA/Dengue) molecule to investigate the binding nature. The binding which was enclosed in a box have number of grid points in x × y × z directions of 40 × 40 × 40 and a grid spacing of 0.35 Å. The docking studies were conducted using AutoDockTools (ADT) version 1.5.4 and finalized by Auto Dock vina program [30]. The docked structures were exposed using Discovery studio software.
Results and Discussion
1H-NMR Spectral Studies and Electron Impact Mass Spectral analysis
In the 1H NMR spectrum of the ligand L1, which is provided in the Figure 1 there is a signal at 9.61 ppm attributed to amido proton of benzanilide group present in the ligand L1 [31]. The signal for azomethine proton appeared as a small peak at 8 ppm [32]. The multiplet in the region 6.05-7.55 ppm represents the protons present in the aromatic moiety.
The Electron Ionization mass spectrum of the Schiff base ligand L1 is given in the Figure 2. The molecular ion M+ peak is present at 470.9364, which is corresponding to the molecular weight of the ligand L1. In addition there is an (M+ + 2) isotopic peak at 472.9053. The peaks at 367.9976, 326.0706, 311.0307, 241.8728, 207.0274, 152.0043 and 91.0319 correspond to various fragments of the ligand.
 |
Figure 1: 1H-NMR Spectra of L1 |
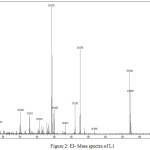 |
Figure 2: EI- Mass spectra of L1 |
FT-IR Spectral Studies
The FT-IR spectrum of the Schiff base ligand L1 and its complexes were represented in the Figure 3. The FT-IR spectra of the ligand L1 exhibits a band at 1592 cm-1, which is attributed to imino stretching frequency of the Schiff base ligand [33]. The strong peak at 3354 cm-1 is the characteristic N-H stretching frequency of the secondary amide group of 4,4ʹ-diaminobenzanilide part of the ligand. To study the binding mode of the ligand to the metal in the complexes, the IR spectrum of the free ligand and its complexes were compared. The band near 1592 cm-1 is shifted to lower frequencies in complexes. This clearly indicates the coordination of the imino-nitrogen of the ligand to the metal center [34]. Further the IR spectra of the complexes show some new sharp signals in the region 467 cm-1, 437 cm-1 and 465 cm-1 for CoL12, NiLl2 and CuLl2 complexes respectively, which corresponds to metal-nitrogen stretching formed by the coordination of imino nitrogen of the ligand with the metal centers.
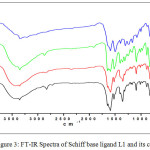 |
Figure 3: FT-IR Spectra of Schiff base ligand L1 and its complexes
|
Electronic Spectral Analysis
The electronic spectra of complexes Co(L1)2, Ni(L1)2 and Cu(L1)2 are recorded in the range of 200 nm-800 nm in DMSO and were provided in Figure 4. The spectral details below 350 nm are similar for all the complexes and they are corresponding to the transitions (π-π* and n- π*) of benzene ring and non-bonding electrons present on the nitrogen of azomethine group in the Schiff base complexes [35]. In the electronic spectra of the cobalt complex, the band around 380 nm is assigned to LMCT [36] transition while the d-d band observed in the lower energy region around 430 nm is assigned to the combination of 2B1g ⟶ 1A1g and 1B1g ⟶ 2Eg transitions. This transition represents a square planar geometry for cobalt complex [37-38]. In the electronic spectrum of the nickel complex, the band around 360 nm is assigned to LMCT transition. Additionally a broad band observed in the lower energy region around 370-410 nm is assigned to d-d (1A1g ⟶ 1B1g) transition. This transition represents the square planar geometry for nickel complex [39]. The electronic spectrum of copper complex shows d-d band at 450 nm, which is attributed to the combination of 2B1g ⟶ 2Eg and 2B1g ⟶ 2B2g transitions. This give the evidence for the distorted square planar geometry for d9 Cu(II) system [40].
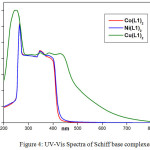 |
Figure 4: UV-Vis Spectra of Schiff base complexes
|
EPR Spectral Analysis
The EPR spectra of copper complex give useful information in studying the metal ion environment. In the EPR spectrum of the copper complex Cu(L1)2 there is an isotropic signal, without any hyperfine splitting, with giso = 2.12 as shown in the Figure 5. When comparing the giso value of the copper complex with the g value of free electron (2.0023) indicate that there is an increase in the covalent nature of the bonding between the metal ion and the ligand molecule [41]. Isotropic lines are usually appeared due to the occupancy of the unpaired electron in a degenerate orbital in square planar geometry.
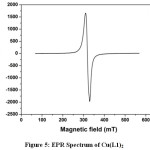 |
Figure 5: EPR Spectrum of Cu(L1)2
|
Molecular Docking with Human DNA Topoisomerase I
Human DNA topoisomerase I was the selective targeting area for synthesizing the anticancer drug [42]. The molecular docking of Co (II), Ni (II) and Cu(II) complexes were performed to determine the binding affinity value and the selected binding residue, along with the sterically suitable conformations. The low value of the binding energy shows the more effective binding affinity between the ‘receptor’(DNA) and the ‘ligand’(complex) molecules. The various conformations of docked molecular complexes were analyzed in terms of binding energy, hydrogen bonding and hydrophobic interaction between receptors and the acceptor. More negative value of the relative binding values suggests that the interaction between the DNA and ligand is so strong, due to the extended aromatic ring. Phenyl ring has higher free binding energy which gives a better binding affinity value comparing with a compound containing bipyridyl ring. In these work mononuclear complexes give better results towards the HDNA. The binding energy values of the Co(II), Ni(II) and Cu(II) complexes were respectively -16.8, -10.4 and -15.1 kcal mol−1 towards human DNA topoisomerase I. This shows that Cobalt(II) and Copper(II) molecule easily bind with the DNA helix and the Nickel(II) complex preferred to bind with the outermost protein’s amino acid residue. The binding values were shown in the table 1, 2 and 3 and the docked images were shown in Figure 6.
 |
Figure 6: (a), (c) and (e) demonstrate the binding of Co(II), Ni(II) and Cu(II) complexes with active site of human DNA topoisomerase I, and (b), (d) and (f) demonstrate the binding of Co(II), Ni(II) and Cu(II) complexes with selective Nucleotide of human DNA topoisomerase I |
Molecular Docking with NS3 Protease-Helicase
NS3 protease-helicase (dengue virus) is a very important target area which should be docked. Cobalt(II), Nickel(II) and Copper(II) complexes exhibit very low binding energy value, it means that complexes having very high binding affinity towards the NS3 protease-helicase. The values of the binding energy in various levels are present in table 1, 2 & 3.The distance between the selected receptor to targeted molecule is also low. The binding energy values of the Co(II), Ni(II) and Cu(II) complexes were -12.4, -13.7 and -13.0 kcal mol−1respectively. The binding interactions were shown in fig.7. From the theoretical point of view these complexes are considered to be a good anti-dengue drug.
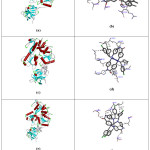 |
Figure 7: (a) (c) and (e) demonstrate the binding of Co(II), Ni(II) and Cu(II) complexes with active site of NS3 protease-helicase, and (b), (d) and (f) demonstrate the binding of Co(II), Ni(II) and Cu(II) complexes with selective Amino acid residue of NS3 protease-helicase |
Table1: Binding interactions of Co(II) complex with human DNA topoisomerase I and NS3 protease-helicase
| Mode |
Affinity (kcal/mol) |
Distance from best mode |
||||
| Human DNA topoisomerase I | NS3 protease-helicase | |||||
|
Human DNA topoisomerase I |
NS3 protease-helicase | |||||
| rmsdl.b. rmsdu.b. | rmsdl.b. rmsdu.b. | |||||
| 1 |
-16.8 |
-12.4 |
0 |
0 |
0 |
0 |
| 2 |
-16.5 |
-12.3 |
3.771 |
9.455 |
2.459 |
10.883 |
| 3 |
-16.2 |
-12.2 |
0.522 |
8.076 |
4.393 |
8.79 |
| 4 |
-15.6 |
-12.2 |
4.114 |
8.625 |
1.405 |
9.713 |
| 5 |
-15.4 |
-12 |
4.148 |
9.703 |
4.273 |
8.645 |
| 6 |
-14.6 |
-12 |
15.172 |
21.499 |
0.596 |
7.523 |
| 7 |
-14.5 |
-11.5 |
4.101 |
9.898 |
4.684 |
8.329 |
| 8 |
-14.4 |
-11.3 |
15.451 |
22.1 |
3.498 |
8.79 |
| 9 |
-14.4 |
-11.1 |
15.378 |
21.17 |
12.94 |
18.217 |
Table 2: Binding interactions of Ni(II) complex with human DNA topoisomerase I and NS3 protease-helicase
|
Mode |
Affinity (kcal/mol) |
Distance from best mode |
||||
| Human DNA topoisomerase I | NS3 protease-helicase | |||||
| Human DNA topoisomerase I | NS3 protease-helicase | |||||
| rmsdl.b. rmsdu.b. |
rmsdl.b. rmsdu.b. |
|||||
| 1 |
-10.4 |
-13.7 |
0.000 |
0.000 |
0.000 |
0.000 |
| 2 |
-9.9 |
-13.4 |
1.841 |
7.560 |
1.573 |
7.663 |
| 3 |
-9.7 |
-12.2 |
5.281 |
12.004 |
31.948 |
38.354 |
| 4 |
-9.5 |
-12.0 |
5.377 |
11.101 |
32.727 |
37.825 |
| 5 |
-9.1 |
-11.9 |
1.954 |
2.421 |
1.7333 |
7.947 |
| 6 |
-8.8 |
-11.7 |
4.640 |
10.252 |
32.315 |
37.338 |
| 7 |
-8.7 |
-11.6 |
7.369 |
13.017 |
46.385 |
51.726 |
| 8 |
-8.6 |
-11.4 |
2.349 |
9.610 |
20.661 |
24.252 |
| 9 |
-8.3 |
-11.2 |
5.571 |
12.245 |
32.817 |
39.072 |
Table 3: Binding interactions of Cu(II) complex with human DNA topoisomerase I and NS3 protease-helicase
| Mode |
Affinity (kcal/mol) |
Distance from best mode |
||||
| Human DNA topoisomerase I | NS3 protease-helicase | |||||
|
Human DNA topoisomerase I |
NS3 protease-helicase |
|||||
|
rmsdl.b. rmsdu.b. |
rmsdl.b. rmsdu.b. |
|||||
| 1 |
-15.1 |
-13.0 |
0.000 |
0.000 |
0.000 |
0.000 |
| 2 |
-14.6 |
-12.3 |
1.229 |
1.495 |
3.964 |
9.445 |
| 3 |
-14.2 |
-12.2 |
10.544 |
18.244 |
2.039 |
9.414 |
| 4 |
-14.2 |
-12.0 |
3.599 |
11.470 |
4.001 |
9.745 |
| 5 |
-14.0 |
-11.8 |
3.636 |
10.036 |
3.795 |
10.126 |
| 6 |
-13.6 |
-11.6 |
1.725 |
9.229 |
32.154 |
38.142 |
| 7 |
-13.1 |
-11.5 |
0.956 |
7.945 |
48.576 |
58.949 |
| 8 |
-13.0 |
-11.5 |
14.412 |
18.972 |
3.690 |
10.115 |
| 9 |
-13.0 |
-11.4 |
10.661 |
17.927 |
4.031 |
10.573 |
Conclusion
A new Schiff base ligand which contains several phenyl rings and its Co(II), Ni(II) and Cu(II) complexes were synthesized. The formation of the ligand and its metal complexes were confirmed by various spectroscopic techniques. The docking studies were carried out using synthesized metal complexes with human DNA topoisomerase I (PDB: 1SC7) and Dengue NS3 protease-helicase (PDB ID: 2VBC) using Auto Dock vina and Discovery studio software. The binding energy values of the Co(II), Ni(II) and Cu(II) complexes were respectively -12.4, -13.7 and -13.0 kcal mol−1 towards NS3 protease-helicase and -16.8, -10.4 and -15.1 kcal mol−1 towards human DNA topoisomerase I, which showed that these types of compounds can be act as an potential Anti-Dengue and Anticancer agent.
Acknowledgement
The authors are thankful to Indian Institute of Technology, Madras, India for EI-mass and EPR spectral analysis and also Dr. S. Gnanavel, Research and Project Centre for Chemical and Biological Science for docking study, Salem, India.
References
- Sigman, D. S.; Mazumder, A.; Perrin, D. M. Chem. Rev. 1993, 93(6), 2295-2316
CrossRef - Jamieson, E. R.; Lippard, S. J. Chem. Rev. 1999, 99(9), 2467-2498
CrossRef - Liu, H. K.; Sadler, P. J. Acc. Chem. Res. 2011, 44(5), 349-359
CrossRef - Leung, M. H. M.; Harada, T.; Kee, T. W. Curr. Pharm. Des. 2013, 19(11), 2070-2083
- Alagesan, M.; Bhuvanesh, N. S. P.; Dharmaraj, N. Dalton Trans. 2014, 43(16), 6087-6099
CrossRef - Shao, J.; Ma, Z. Y.; Li, A.; Liu, Y. H.; Xie, C. Z.; Qiang, Z. Y.; Xu, J. Y. J. Inorg. Biochem. 2014, 136, 13-23
CrossRef - Mjos, K. D.; Orvig, C. Chem. Rev. 2014, 114(8), 4540-4563
CrossRef - Wichapong, K.; Nueangaudom, A.; Pianwanit, S.; Sippl, W.; Kokpol, S. Trop. Biomed. 2013, 30(3), 388–408
- Murphy, F.A.; Mahy, B.W.J.; van Regenmortel, M.H.V. Encycl. Virol. 2008, 140–148
- Mastrangelo, E.; Milani, M.; Bollati, M.; Selisko, B.; Peyrane, F.; Pandini, V.; Sorrentino, G.; Canard, B.; Konarev, P.V.; Svergun, D.I.; de Lamballerie, X.; Coutard, B.; Khromykh, A.A.; Bolognesi, M. J. Mol. Biol. 2007, 372(2), 444–455
CrossRef - Byrd, C.M.; Grosenbach, D.W.; Berhanu, A.; Dai, D.; Jones, K.F.; Cardwell, K.B.; Schneider, C.; Yang, G.; Tyavanagimatt, S.; Harver, C.; Wineinger, K. A.; Page, J.; Stavale, E.; Stone, M. A.; Fuller, K. P.; Lovejoy, C.; Leeds, J. M.; Hruby, D. E.; Jordan, R. .Antimicrob. Agents Chemother. 2013, 57(4), 1902–1912
CrossRef - Noble, C.G.; She, C.C.; Chao, A.T.; Shi P.Y. J. Vir. 2012, 86(1), 438–446
CrossRef - Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. J. Comput. Aided Mol. Des., 2013, 27 (3), 221–234
CrossRef - Bas, D.C.; Rogers, D. M.; Jensen, J. H. Proteins Struct. Funct. Genet. 2008,73(3), 765–783
CrossRef - Li, H.; Robertson, A. D.; Jensen, J.H. Proteins. 2005, 61 (4), 704–721
CrossRef - Irwin, J.J.; Shoichet, B.K. J. Chem. Inf. Model. 2005, 45 (1), 177–182
CrossRef - Greenwood, J. R.; Calkins, D.; Sullivan, A.P.; Shelley, J. C. J. Comput. Aided Mol. Des. 2010, 24 (6-7), 591–604
CrossRef - Greco, M. N.; Hawkins, M. J.; Powell, E. T.; Almond, H. R.; Garavilla, L.; Hall, J.; Minor, L. K.; Wang, Y.; Corcoran, T.W.; Cera, E. D.; Cantwell, A. M.; Savvides, S.N.; Damiano, B.P.; Maryanoff , B. E. J. Med .Chem. 2007, 50(8), 1727–1730
CrossRef - Iijima, K.; Katada, J.; Yasuda, E.; Uno, I.; Hayashi, Y. J. Med. Chem. 1999, 42(2), 312–323
CrossRef - Aoyama, Y.; Uenaka, M.; Kii, M.; Tanaka, M.; Konoike, T.; Hayasaki- Kajiwara, Y.; Naya, N.; Nakajima, M. Bioorg. Med. Chem., 2001, 9(11), 3065–3075
CrossRef - Aoyama, Y.; Uenaka, M.; Konoike, T.; Hayasaki-Kajiwara, Y.; Naya, N.; Nakajima, M. Bioorg. Med. Chem. Lett. 2001, 11(13), 1691–1694
CrossRef - Aoyama, Y.; Konoike ,T.; Kanda, A.; Naya, N.; Nakajima, M. Bioorg. Med. Chem.Lett. 2001, 11(13), 1695–1697
CrossRef - Maruoka, H.; Muto, T.; Tanaka, T.; Imajo, S.; Tomimori, Y.; Fukudaa, Y.; Nakatsuka, T. Bioorg. Med. Chem. Lett. 2007, 17(12), 3435–3439
CrossRef - Wawer, M.; Bajorath, J. J .Chem. Inf. Model. 2010, 50(8), 1395–1409
CrossRef - Thangapandian, S.; John, S.; Sakkiah, S.; Lee, K. L. J. Chem. Inf. Model. 2011, 51(1), 33–44
CrossRef - Lie, M. A.; Thomsen, R.; Pedersen, C. N. S.; Schiøtt B.; Christensen, M. H. J. Chem. Inf. Model. 2011, 51(4), 909–917
CrossRef - Bujacz, A. ActaCrystallogr. D. 2012, 68(10), 1278-1289
CrossRef - Mirza, S.B., Salmas R.E., Fatmi M.Q. and Durdagi S.J, mol graph model., 2016, 66, 99-107.
- Macrae, C. F.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Shields, G. P.; Taylor, R.; Towler, M.; van de Streek, J. J. Appl. Crystallogr. 2006, 39, 453-457
CrossRef - Trott, O.; Olson, A. J. J. Comput. Chem. 2010, 31(2), 455-461
- Abdulghani, A. J.; Mohuee, S.K. J Chem Bio Phy Sci. 2016, 6(2), 579-595
- Patel, K. V.; Bhattacharya, P. K. Ind. J. Chem. 1982, 21(12), 1110-1112
- Singh, D. P.; Vandna Malik; Krishan Kumar; Chetan Sharma ; Aneeja, K.R. Spectrochim. A. 2010, 76(1), 45-49
CrossRef - Raman, N.; Sobha, S. Spectrochim. Acta A. 2012, 85(1), 223–234
CrossRef - Ramesh, R.; Maheswaran, S. J.Inorg.Biochem. 2003, 96(4), 457-462
CrossRef - Ortiz, B.; Park, S. M. Bull. korean Chem.Soc. 2000, 21(4), 405- 411
- Shakir, M.; Nasam, O.S.M.; Mohamed A. K.; Varkey, S.P. Polyhedron. 1996, 15 (8), 1283- 1287
CrossRef - Chem, L. S.; Cummings, S.C. Inorg. Chem. 1978, 17 (9), 2358-2361
CrossRef - Kirchhoff, J. R.; GamacheJr, R. E.; Blaskie, M. W.; Del Paggio, A. A.; Lengel, R. K.; McMillin, D.R. Inorg. Chem. 1983, 22 (17), 2380-2384
CrossRef - Natarajan, C.; Tharmaraj, P.; Murugesan, R. J. Coord. Chem. 1992, 26(3), 205-213
CrossRef - Raman, N.; Dhaveedhu Raja, J.; Shakthivel, A. J. Chem. Sci. 2007, 119(4), 303-310
CrossRef - Pommier, Y. Nat. Rev. Cancer., 2006, 6(10), 789-802
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









