Carbon Mineralization of Agro-Food Waste Compost in Soil Under Controlled Conditions
Sanonka Tchegueni1 , Magnoudéwa B. Bodjona1, Kokou Sabi2 and Moursalou Koriko1
, Magnoudéwa B. Bodjona1, Kokou Sabi2 and Moursalou Koriko1
1Laboratoire Gestion, Traitement et valorisation des Déchets (GTVD), Faculté Des Sciences, Université de Lomé ; BP 1515 Lomé-Togo.
2Laboratoire de Chimie Atmosphérique (LCA), Faculté Des Sciences,Université de Lomé ; BP 1515 Lomé-Togo.
Corresponding Author E-mail: tchegsani@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340321
Article Received on : November 17, 2017
Article Accepted on : February 07, 2018
Article Published : 22 May 2018
Compost mineralization study under controlled conditions is a way to predict the compost behavior in the soil. It permits to know how much compost to bring to the soil to ensure a good amendment and how long the compost will last in the soil. Six composts obtained from agro-food waste are incubated under controlled conditions in order to follow the carbon mineralization. Soil-compost mixtures (100:1) are incubated at 2/3 of soil moisture capacity for 75 days and under ambient temperature (30°C). The soil-compost mixtures released during 75 days 15.96 to 27.52 mg of C-CO2, versus 4.89 mg for the control without compost. This correspond to the carbon potentially mineralizable varing between 25 and 46% of the total organic carbon. The pH of composts and their C/N ratio have effected carbon mineralization.
KEYWORDS:Compost; Organic Carbon; Mineralization; Soil
Download this article as:| Copy the following to cite this article: Tchegueni S, Bodjona M. B, Sabi K, Koriko M. Carbon Mineralization of Agro-Food Waste Compost in Soil Under Controlled Conditions. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Tchegueni S, Bodjona M. B, Sabi K, Koriko M. Carbon Mineralization of Agro-Food Waste Compost in Soil Under Controlled Conditions. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46097 |
Introduction
Compost is an organic amendment that improves the physical and chemical properties of the soil [1] and increases soil biodiversity, because of its microbial flora [2]. Despite these beneficial effects of compost, its use under certain conditions can pose some problems to the soil-plant system.
The mineralization of organic matter in the soil leads to its degradation under favorable conditions of temperature, humidity and pH resulting in the release of minerals (NH4+, K+ Ca2+, Mg2+) essential plants. The study of the mineralization of the compost in the controlled conditions can predict the behavior of the compost in the soil, the quantity useful to a good amendment and the duration of its effect in the soil.
Incubation of compost under controlled conditions and field trial are two complementary methods of studying the effects of compost in the soil often used. In controlled conditions, the incubation consists in mixing the compost with a soil of known characteristics, in a closed enclosure (generally a jar) so as to follow the mineralization of carbon or organic nitrogen. The jars thus formed are kept in the dark and under precise temperature and humidity conditions for a variable duration. Many temperatures are used by authors, 25°C by Huang [3], and 30°C by Cambardella [4]. Moisture content varies between 50% and 75% of soil water holding capacity [1, 5, 6].
Incubation in controlled conditions can be a modeling prior to spreading over larger areas. Mathematical models are used to describe the kinetics of carbon mineralization during incubation [7]. As for the field trial, it allows to know the influence of a contribution of organic matter in the form of compost on the structure of a soil.
This work aims to determine the labile fractions of organic matter and the degradation rates of six compost made from agro-food waste.
Material and Methods
Material
Composts used, obtained from the composting of agro-food waste, were characterized using the parameters mentioned in Table 1.
Table 1: Characteristics of composts
| Basic material | pH | OM (%) | TOC (%) | NTK (%) | C/N | |
| Compost A0 | Citrus wastes | 7,24 | 59,5±0,4 | 31,65±1,11 | 3,78±0,26 | 8,37 |
| Compost A1 | Citrus waste and rock phosphate | 7,20 | 45,0±0,3 | 22,50±1,03 | 2,59±0,17 | 8,69 |
| Compost B0 | Hulls of cottonseed, ash | 9,55 | 57,5±1,1 | 28,79±1,03 | 2,61±0,11 | 11,03 |
| Compost B1 | Hulls of cotton seed, ash and phosphate rock | 9,47 | 48,5±1,4 | 24,75±1,01 | 2,07±0,13 | 11,96 |
| Compost C0 | Sheanut cake | 6,52 | 75,5±1,6 | 38,55±1,10 | 4,20±0,15 | 9,18 |
| Compost C1 | Sheanut cake and phosphate rock | 6,23 | 62,0±2,5 | 34,33±1,35 | 3,40±0,32 | 10,10 |
Notes: OM: organic matter; TOC: total organic carbon; NTK: total nitrogen; C/N: carbon/nitrogen
The soil used for this study is taken from the agro-educational farm in the Agronomy school of the University of Lome (Table 2).
Table 2: Physico-chemical characteristics of the soil
| Parameter | Value |
| pH | 7.73±0.24 |
| TOC (%) | 0.60±0.07 |
| P assimilable (mg/kg) | 13.02±0.99 |
| Exchangeable cations (mg/kg) | |
| Ca | 336.25±16.17 |
| Mg | 27.20±5.50 |
| K | 35.20±2.41 |
| CEC (cmol+/kg) | 1.80±0.09 |
| Granulometry (%) | |
| Coarse sand | 60.45 |
| Fine sand | 25.15 |
| Coarse silt | 2.75 |
| Fine silt | 3.00 |
| Clay | 7.00 |
Methods
25 g of dry soil and 0.25 g of compost were mixed and placed in 75 ml flasks at a humidity adjusted to about 2/3 of the field capacity [8]. Then, the flasks were placed in 500 ml sealed enclosures. Two vials of 20 ml were placed inside each chamber, one containing 10 ml of deionized water to saturate the atmosphere with water, and the other containing 10 ml of sodium hydroxide 1N solution, to fix the CO2 released by the mineralization [9]. The whole is kept at room temperature (30 ° C). All treatments were in three replicates.
At fixed time intervals, the chambers are opened to renew the oxygen while the bottles containing sodium hydroxide 1N solution were removed to be replaced by solution. The CO2 released by the mineralization and captured by the sodium hydroxide 1N solution is precipitated by 5 ml 5% BaCl2. The amount of sodium hydroxide remaining in the solution is titrated with 0.1 N hydrochloric acid (HCl) in the presence of phenophthalein [6]. A control (white) is obtained by carrying out the experiment without adding soil in the corresponding container. The difference in the values observed between each of the treatments (soil-compost mixture) and the control (soil without compost) is considered as the amount of CO2 coming from the compost.
The kinetics of carbon mineralization C-CO2 (t) are adjusted according to the one-compartment model described by the relation [7]:
C-CO2 (t) = C0.(1- e-bt) (E1)
Where: C0 = fraction of mineralizable organic carbon;
t = incubation time in a day;
b = coefficient of mineralization rate of organic carbon per day.
Results and Discussion
Results
The cumulative CO2 released during the incubation of soil-compost mixtures (100:1) in the laboratory for 75 days is shown on Figure 1 and the percentage of mineralized carbon from composts on Figure 2. Kinetics of mineralization during the incubation is adjusted according to the equation (E1). The parameters of these adjustments are shown in Table 3 and Table 4. The correlation coefficients R² between the observed and adjusted values are between 0.947 and 0.998, thus attesting the quality of the adjustments.
The amount of carbon contained in the control, soil without compost, is 148.7 mg. Soil-compost treatments at the dose of 0.25 g of compost (dry) for 25 g of soil (dry) correspond to an exogenous supply of 79.125 mg of carbon for the mixture Soil + A0; 56.25 mg for Soil + A1; 71.975 mg for Soil + B0; 61.875 mg for Soil + B1; 96.375 mg for Soil + C0 and 85.825 mg for Soil + C1, according to the values in Table 1.
Table 3: Adjustment parameters according to C-CO2(t) = Co (1 – e-bt) model of the cumulative amount of mineralized carbon (mg)
| Sample | Co | b | R² |
| Soil (witness) | 4.614 | 0.036 | 0.947 |
| Soil+A0 | 24.563 | 0.022 | 0.994 |
| Soil+A1 | 19.453 | 0.021 | 0.991 |
| Soil+B0 | 35.860 | 0.019 | 0.994 |
| Soil+B1 | 32.519 | 0.020 | 0.997 |
| Soil+C0 | 32.891 | 0.016 | 0.992 |
| Soil +C1 | 33.440 | 0.017 | 0.995 |
Note Co = potentially mineralizable carbon (mg),
b = mineralization rate (per day)
t = mineralization time (day)
Table 4: Adjustment parameters according to C-CO2(t) = Co (1 – e-bt) model of the cumulative amount of mineralized carbon (%TOC).
| Compost | Co | b | R² |
|
A0 |
25.800 | 0.019 | 0.996 |
|
A1 |
27.566 | 0.017 | 0.995 |
|
B0 |
44.801 | 0.016 | 0.996 |
|
B1 |
46.064 | 0.018 | 0.998 |
|
C0 |
31.846 | 0.013 | 0.996 |
|
C1 |
35.677 | 0.014 | 0.997 |
Notes: Co = potentially mineralizable carbon fraction (% TOC),
b = mineralization rate (per day)
t = mineralization time (day)
From Figure 1, the soil-compost mixtures respectively released during 75 days of incubation 19.96; 15.96; 27.52; 25.66; 23.72 and 24.26 mg of C-CO2, versus 4.89 mg for the control without compost; which corresponds respectively to 8.76; 7.79; 12.47; 12.18; 9.68 and 10.34% TOC of the mineralized mixture versus 3.1% for the control without compost. A correlation between the fraction of potentially mineralizable carbon or labile carbon and the C/N ratio of the composts is shown on Figure 3.
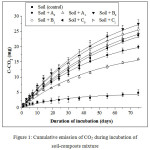 |
Figure 1: Cumulative emission of CO2 during incubation of soil-composts mixture Click here to View figure |
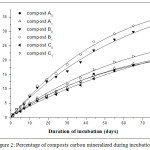 |
Figure 2: Percentage of composts carbon mineralized during incubation |
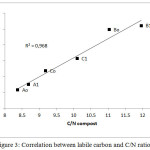 |
Figure 3: Correlation between labile carbon and C/N ratio Click here to View figure |
Discussion
The mathematical description of the dynamics of soil carbon mineralization under controlled conditions is an essential tool for the characterization of soil organic matter and to understand the evolution of carbon in the soil. The carbon fraction of composts potentially mineralizable varies between 25 and 46% of TOC and depends on the composts. It means that 54 to 75% of the TOC, depending on the composts, are refractory to mineralization and can therefore be used to maintain the soil carbon stock for the improvement of its physical properties. Depending on the model of the study and the experimental conditions, after one year, 25.77% TOC of compost A0, 27.51% TOC of compost A1; 44.67% TOC of compost B0; 46.00% TOC of compost B1; 31.57% TOC of the compost C0 and 35.46% TOC of compost C1 will be mineralized. However, soil texture, climatic conditions and the type of agriculture can influence the carbon mineralization of composts in the soil.
For better interpretation, the percentage of exogenous carbon (from composts) mineralized is calculated and shown on Figure 2. These percentages are, after 75 days of incubation, respectively 19.05; 19.68; 31.44; 33.57; 19.54 and 2.57 for composts A0, A1, B0, B1, C0 and C1.
The exponential adjustment parameters, in Table 4, shows that composts B0 and B1 have between 45 and 46% TOC potentially mineralizable, followed by composts C0 and C1 (31 to 36% TOC), then composts A0 and A1 ( 25 to 28% TOC). It should be noted, like Javier [10], that the fraction of potentially mineralizable carbon depends on composted waste. The alkaline nature of composts B0 and B1 would contribute to the high mineralization of their organic matter [11]. Alkaline amendments have been shown to increase the dissolved organic matter content of the soil [12, 13] and to stimulate the activity of soil microorganisms [14], therefore accelerate the mineralization of organic matter. Composts without natural phosphate (A0, B0, C0) are more stable than composts with natural phosphate (A1, B1, C1). Composts with phosphate rock have a slightly higher mineralization rate than composts without phosphate rock. This can be explained by referring to Lompo’s works [15] which showed that phosphate rock in the presence of nitrogen stimulates the activity of microorganism’s biomass and therefore stimulates the degradation of organic matter.
The correlation between the fraction of potentially mineralizable carbon or labile carbon and the C/N ratio of the composts observed, confirms the C/N ratio is a characteristic of the stability of the organic matter [16].
Composts A0, A1, B0 and B1 have mineralization rates close to 0.02 per day while those of C0 and C1 composts are barely approach 0.015 per day. This low level rate can probably be justified by the acidity of these composts (pH = 6.2 and 6.5) because the acidity reduces the mineralization of the organic matter [3, 17].
Conclusion
According to the experimental conditions of this study, the potentially mineralizable carbon fraction of composts varies between 25 and 46% of the total organic carbon; which means that 54 to 75% of the carbon is refractory to mineralization and can therefore be used to maintain the soil carbon stock in order to improve its physical properties. This mineralization is correlated with the C/N ratio of the composts and fast when the compost is alkaline.
However, soil texture, climatic conditions and the type of agriculture have an effect on the mineralization of carbon in the soil.
Acknowledgments
This work was fully supported by « Laboratoire de Gestion, Traitement et Valorisation des Déchets » and « Laboratoire de Chimie Atmosphérique », two labs of the Faculty of Sciences of the University of Lomé. Authors are very grateful to Professor Gado TCHANGBEDJI, Dean of Faculty of Science for his support and improvement of this manuscript.
References
- Tamara C.F., Daniel V. M. J. Environ. Qual. 2006. 35, 183-193.
CrossRef - Tandy S., Healey J.R., Nason M.A.,Williamson J.C., Jones D.L. Environ Pollut. 2009. 157, 690-697.
CrossRef - Huang C.-C., Chen Z.-S. Soil Science and Plant Nutrition 2009. 55, 715–724.
CrossRef - Cambardella C.A., Richard T.L., Russel A. European Journal of Soil Biology 2003. 39,117-127.
CrossRef - Qayyum M.F., Liaquat F., Rehman R. A., Gul M., Hye M.Z., Rizwan M., Rehaman M.Z. Environ. Sci. Pollut. Res. 2007. 24, 26060–26068.
CrossRef - Pedra F., Polo A., Ribeiro A., Domingues H. Soil Biology & Biochemistry 2007. 39, 1375-1382
CrossRef - Annabi M., Bahri H. and Latiri K., Biotechnol. Agron. Soc. Environ., 2009. 13 (3), 401-408.
- Tchegueni S., Kili K. A., Bodjona M.B., Koriko M., Hafidi M., Baba G., Tchangbedji G. Int. J. Biol. Chem. Sci. 2012. 6(3), 1381-1389.
- Som M.-P., Thèse de Doctorat Université de Poitiers, France 2006.
- Javier D.S., Thèse de Doctorat, INP-Toutouse, France 2005.
- Kalbitz F., Solinger S., Park J.H., Michalzik B., Matzner E., Soil Science 2000, 165 (4), 277-304.
CrossRef - Grybos M., Davranche., Gruau G., Petitjean P., Pédrot M., Geoderma, 2009. 154 (1-2), 13-19.
CrossRef - Li Z.P., Han C.W., Han F.X., Geoderma, 2010. 157 (3-4), 206-213.
CrossRef - Xue D., Huang X., Yao H., Huang C., Journal of Environmental Sciences, 2010. 22 (8), 1253-1260.
CrossRef - Lompo F., Segda Z., Gnankambary Z., Ouandaogo N., Tropicultura, 2009. 27 (2) ,105-109.
- Bernal M.P., Alburquerque J.A., Moral R., Bioresource Technology 2009. 100, 5444–5453.
CrossRef - Fabre B., Kockmann F., Fourrages 2006. 185, 103-122.

This work is licensed under a Creative Commons Attribution 4.0 International License.









