Acid free Cr(Vi) Reduction in Contaminated Ground Water Collected from COPR Dump Site
Vanitha Murugaiyan, S. Selvaraj and P. Kamatchi Selvaraj
and P. Kamatchi Selvaraj
Department of Chemistry, Government Arts College for Men (Autonomous), Nandanam, Chennai- 600035, Tamil Nadu, India.
Corresponding Author E- mail: pethkams64@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340318
Article Received on : December 28, 2017
Article Accepted on : January 08, 2018
Article Published : 04 May 2018
Present study explore the possibility of reducing toxic Cr(VI) to Cr(III) without adding acid externally to the level of regulatory norms. Trial experiments were carried out with standard solution containing Cr(VI) of 1976 mg/L to reveal the suitability of SnCl2 as reducing agent for hazardous hexavalent chromium in the absence of mineral acid. Under the same conditions contaminated ground water from COPR dump site was examined. Complete reduction of Cr(VI) and level of total Cr to discharge limit were observed within fifteen minutes for both simulated and contaminated water. This green chemistry approach and lower time duration for reduction is not reported earlier. The efficacy of the process is ascertained by analysing the other important heavy metals like Ni, Cu, Pb and Zn using AASP. Level of chloride, sulphate, BOD, TDS and pH of the treated water were also recorded. Results imply that SnCl2 effectively reduces mobile hexavalent chromium to harmless Cr(III) in the absence of acid.
KEYWORDS:Contaminated Groundwater; Hexavalent Chromium; Tin Chloride; Reduction and Precipitation
Download this article as:| Copy the following to cite this article: Murugaiyan V, Selvaraj S, Selvaraj P. K. Acid free Cr(Vi) Reduction in Contaminated Ground Water Collected from COPR Dump Site. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Murugaiyan V, Selvaraj S, Selvaraj P. K. Acid free Cr(Vi) Reduction in Contaminated Ground Water Collected from COPR Dump Site. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=45375 |
Introduction
Industrial pollution is one of the evils that challenge the earth, air, water and land. Thus there is a striving need for efforts to manage its natural resources, eco system and bio-diversity from the dumping of hazardous waste on land. Many of the countries in the world are prone to land and ground water pollution that endures from Industrial activity. Developed countries like USA, Spain, Italy, France, UK, and developing countries like India, Chine, Pakistan also have COPR dump site1.
Improper dumping of untreated residue obtained from Chromite ore processing industries significantly contaminate the soil and ground water by leaching Cr(VI) and cause environmental impact to the aquifers and surface water and health problems to the surrounding inhabitants2-3. Much studies have already been done to remediate Cr(VI) contaminant in ground water by physical remediation4, chemical5, electrochemical6 and biological transformation7. Adsorbents synthesized from groundwater treatment residuals, eucalyptus bark, activated carbon manufactured from palm shell with polyethyleneimine, agricultural waste biomass, distillery sludge were admitted for the removal of Cr(VI)8-9. Elimination of Cr(VI) has also been carried out by Phytoremediation10, constructed wetland11 and electrochemical methods12.
The most commonly used technology is the precipitation of metal ions as hydroxides, under appropriate pH conditions13. Precipitation of toxic Cr(VI) by converting it in to Cr(III) using reducing agents like ferrous sulphate heptahydrate and monohydrate, Sodium meta bisulphite, Sodium sulphite, Sodium dithionite, hydrazine, hydroxylamine and Ferrous sulphate have been used14-15. Addition of 1.0% of tin chloride reduces 25mg/L Cr(VI), present in the hydrated cement16.
Industries manufacturing glass use Tin chloride as reducing agent 17-18. Colour pigments, sensitized paper, mordant agent in dyeing unit, soldering flux, plastic based electronic components and tin chemicals manufacturing utilizes tin chloride as one of the reactants. Radiopharmaceuticals used as identifying lyophilized kits contain 99mTc-labelled tracers are being prepared with tin chloride. Visualization of heart, blood, lung and bone using nuclear medicine as diagnostic agents also uses tin chloride 19-21. Preservative and colour retention agent existing in food additives contains tin (II) chloride 22.
The main objective of the present contribution is to find out the effectiveness of SnCl2 for reduction of Cr(VI) to zero level in the contaminated groundwater at TCCL study area located at Ranipet, Vellore, Tamilnadu, India. Findings explore a green chemistry approach for the complete eradication of Cr(VI) using Tin Chloride.
Methods and Materials
Laboratory Studies
The analytical grade SnCl2, HCl and NaOH procured from E-Merck India Ltd., were used as such. High purity distilled water was used for analysis. Adjustment of pH was carried out using analytical grade HCl and NaOH (pH meter 240 Elico L1614). Absorbance measurements were carried out in the spectrophotomter UV – 3200, Lab India at 540nm using diphenylcarbazide and heavy metals were determined on an Atomic Absorption Spectrometer (Shimadzu 6800).
The percentage removal of hexavalent chromium was calculated as
Cr(VI)=[( Ci – Cf)/Ci] × 100
Chromium Contaminated Groundwater
The samples of chromium contaminated groundwater (CGW) was collected from the bore wells located at the TCCL site, Ranipet, Vellore, Tamil Nadu, India. The samples were collected in polypropylene containers. The amount of Cr(VI) was 1976 mg/L. The other parameters measured were turbidity – 25.4NTU, total dissolved solid – 9960 mg/L, electrical conductivity – 15,659 μs/cm, Sodium – 125-140 mg/L, Ammonium -1.5 – 4 mg/L, Sulphate – 2500-2860 mg/L, Hardness of calcium 310-350 mg/L, Hardness of magnesium – 1820-1910 mg/L, Chloride – 595 mg/L and Nitrate – 210mg/L.
Results and Discussion
Treatment of Synthetic Cr(VI) contaminated Water
The concentration of Cr(VI) in the sample was brought to 1976 mg/L by dissolving 5.588 g of K2Cr2O7 in 1000 ml of distilled water23. The pH and reducing agents24 are the two important variables in removing Cr(VI) by reduction and precipitation method. When the two variables are combined together, the net result shows the synergy effect of the reducing agent and pH in the conversion of Cr(VI) to Cr(III). In order to ascertain the individual efficiency of reducing agent and the effect of pH in the elimination of Cr (VI) , the studies were adopted by varying the concentration of reducing agent with and without adjusting the pH externally. The results obtained are presented in Table-1.
Table 1: Reduction of Cr(VI) without adjusting pH in SCW
| Dosage of SnCl2 (mg/L) | pH after addition of SnCl2 | Concentration of Cr(VI) after 15min (mg/L) | % reduction after 15min |
| 200 | 4.6 | 1429 | 27.68 |
| 400 | 3.4 | 1070 | 45.85 |
| 600 | 2.88 | 676.8 | 65.74 |
| 800 | 2.75 | 507.5 | 74.31 |
| 1000 | 2.7 | 308.76 | 83.87 |
| 1200 | 2.66 | 148.55 | 92.48 |
| 1400 | 2.68 | 0 | 100 |
Results implied, incremental effect of reducing agent on reduction of Cr(VI). Additional increase in dosage after 1400mg/L, displayed no significant change in the percentage reduction. Allowing the treated sample for long duration did not show any appreciable change in the conversion of Cr(VI).
Earlier report25 recommended pH 2 as the convenient pH for the reduction of Cr(VI) to Cr(III). Based on this, an approach was made at pH 2 by adding HCl along with SnCl2. The results presented in Table-2 reveals that the presence of acid did not alter appreciably the reducing power of stannous chloride. This is in agreement with the earlier report by Marks Neidle et.al.,26.
Table 2: Conversion of Cr(VI) at pH-2 in SCW
| Dosage of SnCl2 (mg/L) | Amount of Cr(VI) after 15min (mg/L) | % conversion after 15min |
| 200 | 1287.5 | 34.84 |
| 400 | 1046.5 | 47.03 |
| 600 | 614.0 | 68.92 |
| 800 | 410.0 | 79.25 |
| 1000 | 220.8 | 88.82 |
| 1200 | 90.8 | 95.40 |
| 1400 | 0 | 100 |
Comparison of the results presented in Table-1, Table-2 and Fig-1 reveals that the present observation took forward the precipitation studies towards green chemistry approach.
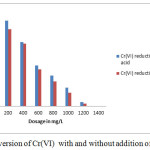 |
Figure 1: Conversion of Cr(VI) with and without addition of acid in SCW |
Treatment on contaminated Cr(VI) groundwater.
The concentration of hazardous Cr(VI) in groundwater indicates not only the toxicity of the solution but also the oxidizing power of the contaminated water. Based on the results obtained from synthetic Cr(VI) water, an experiment on real contaminated Cr(VI) ground water was executed at the optimized concentration of reducing agent.
Table 3: Reduction of Cr(VI) without external addition of acid in CGW (1976mg/L).
| Dosage of SnCl2(mg/L) | pH | Cr(VI) Concentration after reduction | % reduction |
| 200 | 5.2 | 1504 | 23.88 |
| 400 | 4.2 | 1220 | 38.25 |
| 600 | 3.61 | 986 | 50.10 |
| 800 | 3.34 | 608 | 69.23 |
| 1000 | 3.102 | 383.5 | 80.59 |
| 1200 | 3.06 | 85 | 95.69 |
| 1400 | 2.769 | 0 | 100 |
Table 4: Reduction of Cr(VI) without external addition of acid in CGW(1271 mg/L).
| Dosage of SnCl2 (mg/L) | pH | Cr(VI) Concentration after reduction | % reduction |
| 200 | 6.2 | 730 | 42.56 |
| 400 | 4.6 | 429.8 | 66.18 |
| 600 | 3.4 | 193 | 84.81 |
| 800 | 2.9 | 52.7 | 95.85 |
| 1000 | 2.6 | 0 | 100 |
Comparison of Table-3, Table-4 and Figure-2 shows that the amount of stannous chloride reduces with reduction in concentration of Cr(VI) present in untreated water.
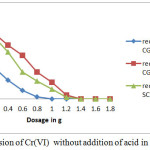 |
Figure 2: Conversion of Cr(VI) without addition of acid in SCW and CGW |
The treated water was analysed for other important parameters and the results obtained are given in Table-5. The amount of Cr(VI) and total Cr in the treated water fall within the regulatory level.
Table 5: Results of the various parameters before and after treatment for CGW at pH-9 precipitation.
| Parameters | CGW | Treated CGW | Disposal standard |
| pH | 6.22 | 7.1 | 5.5-9.5 |
| Cr(VI) (mg/L) | 1976 | 0.00 | 0.05 |
| Total Chromium (mg/L) | 2399.9 | 0.0256 | 2.00 |
| Chloride( mg/L) | 135 | 5494 | 1000 |
| Sulphate (mg/L) | 2526 | 1139 | 1000 |
| BOD (mg/L) | 53 | 13 | 30 |
| Cadmium (mg/L) | 0.0549 | 0.0142 | 2.0 |
| Nickel (mg/L) | 0.2737 | 0 | 3.0 |
| Copper (mg/L) | 0.1200 | 0.0230 | 3.0 |
| Lead (mg/L) | 0.6236 | 0.052 | 0.1 |
| Zinc (mg/L) | 2.8893 | 0.042 | 1.0 |
| Total Dissolved Solids (mg/L) | 4200 | 10000 | 2100 |
Conclusion
The observations in the above results reveal that the elimination of Cr(VI) can be achieved without adding acid externally. This observation has not been reported elsewhere. Further, the level of Cr(VI), total Cr and pH of the discharge water are within the recommended level. Shortest time duration reported in this paper is the first observation reported so far. The high value of TDS can be reduced by Reverse Osmosis Process. To handle the sludge generated in this process, solidification27 method is under investigation.
Acknowledgement
The authors gratefully acknowledge M/s. Tamilnadu Waste Management Limited, certified by National Accreditation Board for Testing and Calibration Laboratory, SIPCOT Industrial Estate, Gummudipoondi – 601201, Tamilnadu, India for their kind permission to carry out all the analytical studies on free of cost.
References
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. J. Hazard. Mater. 2013, 250, 272-291.
CrossRef - Tamma Rao,G.; Gurunadha Rao,V.V.S.; Ranganathan, K. J. Earth Syst. Sci. 2013, 122, 855–867.
CrossRef - Gebre, A.E.; Demissie, H.F.; Mengesha, S.T.; Segni, M.T. J. Environ. Anal. Toxicol. 2016, 6, 363-369.
- Chang, Y.Y.; Lim,J.W.; Yang, J.K. J. Ind. Eng. Chem. 2012, 18, 188-192.
CrossRef - Chrysochoou, M.; Johnston, C.P.; Dahal, G. J. Hazard. Mater. 2012, 201, 33-42.
CrossRef - Barrera-Diaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. J. Hazard. Mater. 2012, 223, 1–12.
CrossRef - Jeyasingh, J.; Somasundaram, V.; Ligy Philip.; Murty Bhallamundi. Chem. Eng. J. 2011, 167, 206-214.
CrossRef - Chi-Chuan Kan.; Aldwin, H.; Kim Katrina, Rivera,P.; Sustain. Environ. Res. 2017,27, 163-171.
CrossRef - Saha,R.; Saha, I.; Nandi, R.; Ghosh, A.; Basu, A.; Ghosh, S.K. Can. J. Chem. Eng. 2013, 91, 814-817.
CrossRef - Doumett,V.S.; Lamperi, J.; Checchini, L.; Azzarello, E.; Chemosphere, 2008, 72, 1481-1490.
CrossRef - Tadesse Alemu Terfie, Seyoom Leta Asfaw. Afr. J. Environ. Sci. Technol. 2015, 9,420-427.
CrossRef - Anirban Kundu.; Bhaskar Senguptha, M.A.; Hashim Ghufran Redzwan. J. Tai. In. Chem. Eng, 2015, 57, 91-97.
CrossRef - Sergioh, Pezzin.; Jose, F ,Lugo Rivera.; Carlo, H, Collins.; Kenneth, E, Collins.; J. Braz. Chem. Soc. 2004, 15, 1678-4790.
- Faith sevim.; Derya Demir. Investigation of reduction kinetics of Cr2O7and in FeSO4 solution, Chem. Eng. J. 2008,143, 161-166.
CrossRef - Vanitha Murugaiyan.; Sehar.T.; Selvaraj.S.; Kamatchi Selvaraj. P. Asian J. Chem. 2018, 30, 620-624.
CrossRef - Prameena Sheeja,J.L; Removal of Chromium with the Complexing Agents from Industrial Effluents, Orient. J. Chem. 2016, 32, 2209-2213.
CrossRef - Graf G.G. Ullman’s Encyclopedia of Industrial Chemistry. 1987, 27, 79–81.
- Gaver C.C. Kirk-Othmer Encyclopedia of Chemical Technology, 1997, 4, 105–122.
- Francis, M.D.; Tofe, A.J.; Hiles, R.A.; Birch, C.G.; Bevan, J.A.; Grabenstetter, R. J. Int. J. Nuc. Med. Biol. 1981, 8, 145–152.
CrossRef - Popescu, H.I.; Lessem, J.; Erjavec, M.; Fuger.G,F. Eur. J. Nucl. Med.1984, 9, 295–299.
- Rao, S.A.; Knobel, J.; Collier, B.D.; Isitman,A.T. J. Nucl. Med. 1986, 27, 1202–1206.
- ATSDR Toxicological profile for tin and compounds (update). Draft for public comment. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. 2003.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, The American Water Works Association (AWWA) and the Water Environment Federation (WEF) Publication. 2006.
- Mottalib, M.A.; Somoal, S.H.; Islam, M.S.; Alam, M.N.; Nurul Abser, M.N. Int. J. Current Research. 2015, 7, 16795-16798.
- Marks Neidle.; Joshua C. Witt. A Contribution to Colloid-Chemistry. J. Am. Chem. Soc. 1916, 38, 47–52.
CrossRef - Gang Wang, Qing Chang.; Mingyue Zhang, Xiaoting Han. React. Funct. Polym. 2013, 11, 1439- 1446.
CrossRef - Sehar.T.; Vanitha Murugaiyan,.; Selvaraj.S. Ind. J. Sci. Tech. 2016, 9, 20. ISSN : 0974-5645 (Online).

This work is licensed under a Creative Commons Attribution 4.0 International License.









