Effect of TiO2 For Generation of H2/O2 Gases, Based on Splited water and UV as Inisiator
Minto Supeno and Rikson Siburian
and Rikson Siburian
Department of Chemistry, Faculty of Mathematics and Natural Sciences-Universitas Sumatera Utara Jl. Biotekhnologi, No.1 Padang Bulan-Medan (82500)-Indonesia.
Coresponding Author E-mail: mintosupeno09@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340247
Article Received on : January 04, 2018
Article Accepted on : February 15, 2018
We reported that Na-bentonite performance which was obtained at Kecamatan Padang Tualang, Kabupaten Langkat. The purposes of this research are to produce pillaried clay base on bentonite material and to study of hydrogen and oxygen formation as well as performance of pillaried clas as catalyst and cocatalyst to splited water. The TiO2-bentonite performance was characterized with XRD, FTIR, BET, SEM, and hydrolysis of water to form hydrogen and oxygen. The results show that etched TiO2–bentonite which was heated at 450°C has wide surface area (92.01 m2/g) and the porous volum 0,044 cm3/g, and showed that the total hydrogen and oxygen gases produced was 78.5 % after 4 days. It is higher than non-etched TiO2–bentonite (60.4 %). It concludes that TiO2-bentonite may be used as a co-catalyst to produce hydrogen.
KEYWORDS:Pillaried Clay; Bentonite; TiO2; H2-O2 Formation; Splitting Water
Download this article as:| Copy the following to cite this article: Supeno M, Siburian R. Effect of TiO2 For Generation of H2/O2 Gases, Based on Splited water and UV as Inisiator. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Supeno M, Siburian R. Effect of TiO2 For Generation of H2/O2 Gases, Based on Splited water and UV as Inisiator. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44945 |
Introduction
There are two kinds of material pillared bentonite in north sumatera, wyoming clay and non wyoming clay, both have SiO2/ Al2O3as a main composition with (4 – 6 : 1) ratio. Bentonite is a general name from the kind of clay which use to adsorbing colour, oil, grease and waxes. Paled soil is a silicate with a various composition, with SiO2 and Al2O3 as a main composition which contains water and chemically bonded. Be sides of two compounds above bentonite is also containing CaO, MgO, Fe2O3, Na2O and K2O. According to Davis and Masser Theory, active power will be influenced by the different between quantity of SiO2 dan Al2O3. Soil which have a big ratio of SiO2 and Al2O3, best for adsorption. Meanwhile, soil which less ratio of SiO2 and Al2O3, less capability in adsorption. The right ratio of SiO2 and Al2O3for a good bentonite to adsorption is 5 – 6 : 1, and it has a wide surface1,10.
Bentonite has a strong colloid power, it will be wyoming if mixed with water. Dried bentonite is light-yellow – green with molecular weigh between 2,4 – 2,8 g/mm3 and melting point between 1330 – 1430°C. Natural bentonite generally contain little calcite, carbonate, gypsum and quartz. Surface and pores natural bentonite can be enlarged with the activation of chemical and physical techniques2, or by using pillaried elements Zr, Ti, Fe, Na, Ca through intercalation technique and calcination at 450°C to produce pillared bentonite called photocatalyst powder3,5.
Semiconductor photocatalyst powder has been widely studied, found that the activity of the photocatalyst is getting better with decreasing particle size causes increased surface area. A decrease in particle size between 5-10 nm causes changes in energy band structure of the semiconductor becomes known as a side effect Kwantum. Further research has been done to produce photochemical of various sizes and shapes, particles of semiconductor kolokogenide such as CdS, ZnS, CdSe, GeSe, ZnSe and oxide semiconductors of the type ZnO, Fe2O3, TiO2 has been widely used to photocatalysts for producing hydrogen from water4,9.
Principle alter the surface and pore bentonite is by dissolving the metals contained in the pores of bentonite with an acid and metal is already dissolved so the pores become wider. Another method to expand the pores by pillaring, in this case pores bentonite containing metal Na and K intercalated with metal cations diameter is larger so that the pores inflates, then calcined at a temperature of 300-500°C4. The metals will form oxides bonded to inter-layers, resulting pillared bentonite. Through this technique would be great porosity bentonite, metal oxides as pillared agent can be used for the catalyst.
In this research intercalation bentonite pores using TiO2 and calcination temperatures of 300-500°C to produce pillared bentonite – TiO2. In isolator part ie oxides can be etched to remove oxides by using a mixture of HF/H2O/NH4F or HF/HNO3/H2O or by using CF4/H2 which produces silicon layer that is free from oxide and silicon is then etched with a solution of HF/HNO3/CH3COOH/I2 so that the silicon will be dissolved. The amount of surface area that is produced depends on the time used for etching. If the time spent too long SiO2 or Si late at all and thus it is not expected that the time used for etching needs to be controlled5,6.
If the etching technique is achieved then the surface and pores pillared bentonite become larger which allegedly produce macropores pillared bentonite. Pillaring by using TiO2 and etching bentonite silicate can change the physical and chemical properties, increase the basal spasing (D001), specific surface area, total volume, surface acidity and decrease the average pore spokes.
TiO2 pillared bentonite can be used as catalysts in the manufacture of hydrogen gas and oxygen from the water, so in this study the researchers are interested in examining the provision of this pillared bentonite as a catalyst.
Natural bentonite has 60% of its silicon content, to provide this material as a catalyst it is necessary to increase the surface area and pore volume by way of intercalation with TiO2 and be TiO2 – pillared bentonite. Titania metal oxide is a material that is sensitive to light and either be a photochemical catalyst. If TiO2 pillared bentonite do etching with chemicals then etched pillared bentonite can be co-catalyst. So that needs to be studied preparing a catalyst sensitive to sunlight from natural bentonite and whether TiO2 pillared bentonite that has been etched can be as a co-catalyst manufacture hydrogen and oxygen gases from water7.
Experimental
Provision of Na-Bentonite
One hundred grams of bentonite clay (3.3) is then dispersed into the 1.5 l NaCl 1 M and submergeded for 1 week in which every two days NaCl solution is replaced with a new one. At every replacement of NaCl stirring for 24 hours by heating to 60-70°C for 4 hours, then after its precipitate filtered washed with demineralized water until free of chloride ions, evidenced by a negative silver nitrate test. Filtering is done using vacuum filters and bentonite obtained dried in an oven of 100°C, after dry crushed and sieved using a 100 mesh sieve.
Furthermore, bentonite is saturated using 6 M NaCl while stirring for 24 hours, then filtered by vacuum filtration and washed with distilled water until free of chloride ions with negative AgNO3 test. Then dried in an oven at 100°C. After dried crushed into powder and then sieved using a 100 mesh sieve. Results saturation of bentonite clay is called Na-bentonite.
Activation of Na-Bentonite with Acid
Each 35 grams of Na-bentonite dispersed into 150 ml solution of sulfuric acid 0,5; 1; 1.5; and 2.0 M while stirring with a magnetic stirrer for 6 hours. Then allowed to stand for 24 hours and then filtered by vacuum filtration and washed with hot distilled water until free of sulfate ions. This is indicated by a negative test against BaCl2. Activated acid Na-bentonite is then dried in an oven at 100°C. After dried crushed into powder sieved using a 100 mesh sieve size. This product is called the Na-bentonite, the product was tested by X-ray diffraction and FT-IR.
Intercalation and pillarization Na-bentonite
Weighed each 30 grams of Na-bentonite clay and dispersed into 1.5 l of deionized water (aquabidest) and stirred with a magnetic stirrer for 6 hours. Then into each Na-bentonite poured piecemeal solution of 0.82 M TiCl4 while stirring with a magnetic stirrer for 10 hours. Results of intercalation separated by vacuum filtration and then washed several times with deionized water until free of chloride ions. Washing stopped if the filtrate is tested with silver nitrate does not form a white precipitate. Bentonite clay that has been intercalated with TiCl4 dried in an oven at 100°C. Once dried crushed into powder and sieved with a 100 mesh sieve then calcined at 350°C. This product is called TiO2-bentonite (Bask, 1992, Long and Yang, 1999).
Etching of TiO2 pillared Bentonite
TiO2 pillared bentonite calcined at a temperature of 400°C is taken as much as 20 g, then put in a plastic container. Etcher solution is then added (a mixture of: 3ml HF + 5ml HNO3 (p) + 3ml CH3COOH (glacial) / I2 of 0.3 g / 250 ml H2O). Then stirred using a plastic stirrer for 10 minutes, then the precipitate is separated from the solution by decantation using a plastic pipette. The precipitate was then dispersed in aqua bidestilate then neutralized pH, decanted using a plastic pipette. Etching products are divided into 3 parts, each furnaced at 400, 450, 500°C for 1 hour. Then the lower heated products were analyzed by SEM and Surface Area analyzer.
The results of SEM photos of surface area analyzer indicates that the product is heated at a temperature of 450°C has the most extensive surface area and will be used to test the catalyst/co-catalyst in water.
Producing of Hydrogen and Oxygen Gas from Water Using the catalyst / co-catalyst TiO2 pillared Bentonite by UV irradiation Wavelength 180 nm
Bentonite from (3.7) and (3.8) weighed as much as 4 g, then put in a pumpkin that has been filled in 10 ml of distilled water and stirred for 10-15 minutes then measured the pH. Pumpkin is connected with a thermometer and three branch pipes are connected to a manometer. Subsequently irradiated with ultraviolet at a wavelength l = 180 nm irradiation carried out for 1-5 days and observed no changes in the manometer. Manometer result of changes in total gas pressure can be calculated total gas (%).
Testing of Hydrogen Gas from Water As a result of irradiation of UV Wavelength 180 nm
Testing hydrogen gas formed from water (distilled water) using a catalyst TiO2 pillared bentonite and TiO2 bentonite etched qualitatively:
As a result of UV radiation at a wavelength of 180 nm on the third day occurs the gas bubbles from the bottom of the pumpkin that leads upwards and more and more so that shifts the pressure manometer.
On the fourth day of gas bubbles produced more and more pressure manometer changed.
The gas produced is tested by flowing gas on the metal oxide powder CuO simmering water vapor will be formed at the pipe wall of this test indicate the presence of hydrogen gas.
The hydrogen gas and oxygen generated from water is detected by a sensor digital hydrogen and oxygen gas.
Results and Discussion
The composition of bentonite of Padang Tualang district, Langkat is shown in Table 1.
Table 1: Composition of bentonite of Padang Tualang district, Langkat
| Elements | Composition (%) |
| SiO2 | 61.02 % |
| Al2O3 | 15.21 % |
| Fe2O3 | 4.89 % |
| TiO2 | 0.62 % |
| CaO | 2.08 % |
| MgO | 1.94 % |
| K2O | 0.46 % |
| Na2O | 3.45 % |
| Missing incandescent | 10.31 % |
| Water content | 7.07 % |
Based on the analysis of the composition of bentonite Kabupatan langkat then bentonite above include Na-bentonite type or swelling, bentonite is so dried in an oven at 100°C and crushed and sieved to 100 mesh sieve. Bentonite is then soaked in 1 M NaCl for 1 week, so going enrichment of Na-bentonite after forming sodium bentonite then put into a 100°C oven until dry and after dry sieved to 100 mesh sieve. The last step enrichment sodium bentonite made by dispersing the Na-bentonite 6 M NaCl solution or saturated NaCl for 24 hours, then washed and dried 100°C, this material is called Na-bentonite.
Na-bentonite subsequently dispersed into several solution of sulfuric acid 0.5; 1; 1.5; 2 M stirred with a magnetic stirrer, activation carried out for 24 hours, filtered by vacuum filtration and then dried in an oven. Activation is aimed to increase the distance between Na-bentonite layer so that it becomes larger.
Once the distance between the layer of Na-bentonite new enlarged carried intercalation and pillarization where activated Na-bentonite dispersed 0.82 M TiCl4 complex solution while stirring with a magnetic stirrer for 18 hours. The results of this intercalation separated by a vacuum pump, intekalasion purpose to enter into the complex Ti distances between the bentonite layer, then calcinated at 350°C to form a more solid oxide pillars.
Analysis was done by X-ray diffraction, using Cu powder radiated by Ka, each 2 grams of TiO2 pillared bentonite and activated clay filled into a sample and then made diffractogram with l = 1.5425 Å.
Based on the results of measurement of basal spacing (D001) there was an increase in basal spacing on the TiO2-pillared bentonite using acid activation of 0.5 and 1.5 M while those using TiO2 pillared bentonite activation damaged. It can be seen from the X-ray diffraction data. The increase in basal spacing will be followed by an increase in surface area, increased porosity, and total volume.
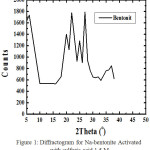 |
Figure 1: Diffractogram for Na-bentonite Activated with sulfuric acid 1.5 M |
Table 2: The Na-bentonite is characterized by peaks at 2-theta
|
Type of Mineral |
d ( A) |
2- Theta |
| Na–Bentonite | 14.9113.884.703.04 | 5.926.3618.8429.28 |
| Kaolinit | 8.273.572.32 | 10.6824.8838.68 |
| Quartz | 4.072.51 | 21.8035.68 |
| Mica | 3.34 | 3.34 |
Based on Table 2, the Na-bentonite is characterized by peaks at 2-theta ie: 5.92; 6.36; 18.84; 29.28 with basal spacing d (A) respectively: 14.91; 13.88; 4.70; 3.04 and other peaks are kaolinite, quartz, mica means bentonite has not been enriched so there are still impurities.
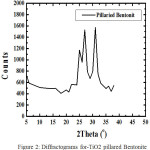 |
Figure 2: Diffractograms for-TiO2 pillared Bentonite |
From this diffractogram (Figure 2) can be given information about changes in the angle theta 6 changes the distance between layers of Na-bentonite into a pillared bentonite-TiO2 for observation or pillared bentonite changes in the angle theta 0-5. From Figure 4.1 and 4.2 have been changes in peak intensity and changing the distance between layers D001.
From the X-ray diffraction data above (Figure 1 and 2) can be determined the distance between layers, as well as identification in identifying the types of clay mineral, to calculate the distance between layers (d) mineral bentonite can be used formula Bragg:8
nλ = 2 d Sin θ
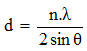
where, d = the distance between the fields of atomic crystals
λ = wavelength (1 Å = 10-10 m)
θ = angle of diffraction
n = order of diffraction
(a) The distance between layers (d) for the Na-bentonite
n = 1
λ = wavelength (1 Å = 10-10 m)
2 θ = 5.920; θ = 2.960
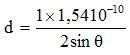
d = 14.917 Å
(b) TiO2 pillared Bentonite using 1.5 M sulfuric acid can be calculated as follows:
n = 1
λ = 1.54 x 10-10 m
2 θ = 5.920; θ = 2.960
d = 16.9807 Å
Further changes in the distance between layers (Dd) are:
(Δd) = d (b) – d (a)
= 16.980 to 14.916
= 2.063 Å
Based on X-ray diffraction analysis then by intercalation and pillarization add, increase the porosity of the basal spacing = 2.06 Å.
Table 3: Basal spacing (D) of the pillared Bentonite Using Various Sulphuric Acid Concentration
| Concentration H2SO4 (M) | Basal spacing d001 |
| Na–Bentonit0.5 M | 14.916715.6566 |
| 1.0 M | 13.8857 |
| 1.5 M | 16.8857 |
| 2.0 M | 9.0554 |
Based on data (Table 3), then pillarization has succeeded at a concentration of 1.5 M H2SO4 with d = 16.8857 Å, so pillarization TiO2 has increased the distance between the layers of d = 2.0633 Å. Data analysis is then performed using FT-IR
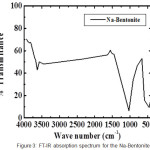 |
Figure 3: FT-IR absorption spectrum for the Na-Bentonite |
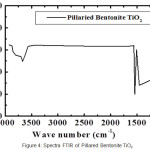 |
Figure 4: Spectra FTIR of Pillared Bentonite TiO2 |
Wave numbers that indicate the presence of Ti is the wave number as follows:
Table 4: Analysis of FTIR
| Group | Absorption cm-1 |
| Impure SiOH | 3898 |
| TiOH on the edge of grid | 3701 |
| TiOH Bridge in (110), H2O Adsorption | 3445 |
| TiOH Terminal in (110) | 3622 |
| TiOH Acid Bridge | 3680 and 3620 |
| TiOH in (100) | 3587 |
| TiOH in (110) | 3445 |
| TiO2 | 796 |
In the FT-IR spectra have seen a shift wave number around 798 cm-1 to 794 cm-1 is due to the pillared bentonite pillaring process is well established in dispersion 1.5 M sulfuric acid, it is adjusted to the data of X-RD confirming the intercalation and pillarization perfect and this is the best for the pillars.
From the data by calculating the surface area analyzer surface area obtained the results as shown in Table 5.
Table 5: Surface Area and Pore Volume Total of bentonite pillared on condition Acids by Using Equation BET
| Sulfuric Acid Concentration (M) | Surface Area (m2/g) | Total Pores Volume (cc/g) |
| 0.5 | 83.3018 | 0.0415 |
| 1 | 86.8939 | 0.0435 |
| 1.5 | 89.0563 | 0.0445 |
| 2 | 88.7607 | 0.0443 |
Based on the data of three X-RD, FT-IR and visible surface at a concentration of 1.5 M sulfuric acid good for intercalation in pillarization produce physical changes in basal spacing, surface area and total pore volume increases.
Furthermore TiO2 pillared bentonite activated in best H2SO4 etched using a mixture (28 ml HF + 170 ml H2O + 113 g NH4F) for 2-10 minutes for etching the oxide on silica and make a lot of holes (H+) on silica, then etched using solution (1 ml 5 ml HF + HNO3 + 2 ml CH3COOH + 0.3 g I2 / 250ml H2O) for 5-10 minutes for etching silicon further heated to 400, 450, and 500°C for 1 hour. With such technique will produce pillared bentonite macropore and reproduce (H+).
Based on this data (Table 6) then etching increases the surface area of the surface area of Na-bentonite 89.0563 m2 / g increased to 92.0123 m2 / g so that the average increase surface area of 2.956 m2 / g, this result has been satisfactory. These results were further tested using analysis of surface area (BET), the result is as follows:
Table 6: Surface Area TiO2 pillared Bentonite which has been etched on the Different Temperatures
| Temperature (o C ) | Surface Area (m2/g) | Totat Pores Volume (cc/g) |
| 400 | 90.2387 | 0.0446 |
| 450 | 92.0123 | 0.0444 |
| 500 | 91.1255 | 0.0444 |
Furthermore TiO2 pillared bentonite photographed SEM showed that the surface be great.
 |
Figure 5: SEM image for Na-Bentonite |
The SEM images (Figure 5) shows the surface is still smooth (white picture) consisting of silicate which is a surface that has not been etched by chemical reagents.
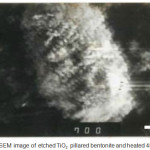 |
Figure 6: SEM image of etched TiO2 pillared bentonite and heated 450°C |
Figure 6 shows the number of holes on the surface of the silicate almost entirely on TiO2 pillared bentonite that has been etched. This surface could imply that the TiO2 pillared bentonite has a lot of holes etched then occurs in the silicate external and internal possibilities.
Preparation of Na-Bentonite
Bentonite samples from the District Padang Tualang, Langkat that have not done enrichment bentonite, made into Na-bentonite produce basal spacing D001 = 14.917 Å, whereas in theory Na-bentonite its basal spacing = 9.8 Å. This means Na-bentonite absorbs water from the humidity so that the measurement time X-ray diffraction to be great. From the X-ray diffraction data (Figure 4.1) clearly show Na-bentonite which still contains koilinit, quartz and mica. Na-bentonite can be observed a peak at an angle of 0-5 theta, at the height of this is the identity of the Na-bentonite.
Intercalation and pillarization
Na-bentonite subsequently soaked with sulfuric acid of 0.5-2 M and intercalated using Ti2+ then pillared at 350°C. The calcination is useful to form the pillars of oxide on bentonite. Thus forming a TiO2 pillared bentonite. For the identification of pillared bentonite seen from the X-ray diffraction data at an angle 0-5 theta, which turned into a basal spacing 16.9807 Å.
This means that the manufacture of the pillared bentonite has been successful in increasing the basal spacing, surface area and pore volume. The study of literature obtained basal spacing of 28.3 Å. This could occur because of the purity of the bentonite is used, meaning that bentonite material is different then produced basal spacing on different pillars.
Etching TiO2 pillared Bentonite
TiO2 pillared bentonite subsequently etched using a chemical etcher to reproduce hole (h+). Hole in silicate form marked changes in surface area and pore volume of the original. Also, based on the SEM image surface becomes more rugged than ever before.
As a result of ultraviolet radiation l = 180 nm, the hydrogen bonding of water will be released and oxygen from water interacting with metal oxides TiO2 and hydrogen from the water molecule will interact with silica. These interactions can lower the activation energy of water molecules. Ultraviolet light enter the pores of bentonite by SiO2 ultraviolet light is converted to shortwave resulting hydrogen and oxygen molecules to break up.
Testing of gas generated a total of 78.5% using pillared bentonite etched, while those using TiO2-pillared bentonite gas generated as much as 60.4%.
Conclusion
Base on the results, we conclude that TiO2 pillared Bentonite made from sodium bentonite types can increase the basal spacing, surface area and total pore volume. SiO2 of TiO2 pillared bentonite etched going hole so that the silica is a hollow volume, so it can be as a co-catalyst. Finally, TiO2 pillared Bentonite is made in an atmosphere of 1.5 M sulfuric acid can be used as a catalyst manufacture of hydrogen gas.
Acknowledgments
This study was supported by TALENTA Research of Universitas Sumatera Utara No. 118/UN 5.2.3.1/PPM/KP-Talenta USU/2017.
References
- Bean, K. E., IEEE. Trans Electron Devices, 1978. 25, 1185.
CrossRef - Bradley, S.M., Kydd, R.A., Yamdagni, R., and Fyfe, C. A., 1992, 2, Van Nostrand Reinhold, New York
- Al-Qunaibit, M.H., and Mekhemer, W. K., J. Colloid and Interface Science, 2004, 2, 1 – 6.
- Burch, R., Pillared Clay, 1997, Elseiver Science Publishier Amsterdam, 283 – 297
- Cool, P., and Vansant, E. F., Pillared Clays: Preparation, Characterization, and Application”, Laboratory of Inorganic Chemistry, 2002, Department of Chemistry
- Anthony, R. W., Solid State Chemistry and Its Applications, 1990, John Wiley and Sons, New York
- Brunaeur, S., Emmet, P.H., and Teller, E., J. of Amateur Chemistry Society, 1938, 60, 309 –319.
CrossRef - Barksdale, J., Titanium, 1966, 2, Ronald, New York
- Brawn, G., and Min. S. D. The X-Ray Identification and Crystal Structures of Clay Mineral, 1972. Elseivier, Netherland
- Cullity, B. D., Element of X-Ray Diffraction”, 1998, 2, Addison Wesley Publishing Company, Inc., Sydney
- Bask, Introduction to Colloid Chemistry Interscience, 1992, New York.
- Barrer, R. M., Zeolites and Clay Minerals as Sorbent and Molecular Sieve, 2002, Academic Press, London.

This work is licensed under a Creative Commons Attribution 4.0 International License.









