Synthesis and Characterization of Natural Ca(OH)2/KF Superbase Catalyst for Biodiesel Production from Palm Oil
I. F. Nurcahyo1,2 , Karna Wijaya1, Triyono1, Arief Budiman3 and Yun Hin Taufiq-Yap4,5
, Karna Wijaya1, Triyono1, Arief Budiman3 and Yun Hin Taufiq-Yap4,5
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Gadjah Mada University, Sekip Utara BLS 21, Bulaksumur, Sleman, Daerah Istimewa Yogyakarta 55281, Indonesia.
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, Sebelas Maret University, Jl. Ir. A. Sutami 36A, Kentingan, Surakarta 57126, Indonesia.
3Department of Chemical Engineering, Faculty of Engineering, Gadjah Mada University, Jl. Grafika No. 2 Yogyakarta 55281, Indonesia.
4Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia.
5Catalysis Science and Technology Research Centre (PutraCAT), Faculty of Science, University Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia.
Corresponding Author E-mail: ifnurcahyo@staff.uns.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340218
Article Received on : November 12, 2017
Article Accepted on : February 10, 2018
The natural Ca(OH)2/KF superbase catalyst was synthesized by grinding and calcinations with a rapid thermal annealing method. It was applied to transesterification of palm oil into biodiesel. The effect of molar ratio Ca(OH)2 to KF and calcinations temperature on the catalyst character and the catalytic activity was investigated. The catalysts were characterized by XRD, FTIR, CO2-TPD, surface area, and TGA. The results indicated that natural Ca(OH)2 reacted to KF in forming monoclinic and orthorhombic KCAF3. The monoclinic KCF3 was produced by reaction between Ca(OH)2 and KF, where as the orthorhombic KCaF3 was produced by reaction between CaCO3 impurities and KF. The best catalyst was natural Ca(OH)2/KF with molar ratio of Ca(OH)2 to KF 0.8:1 and calcinations at 500oC. The catalyst was able to convert 97.6 % oil into biodiesel within 1 minute at 5 % catalyst, molar ratio oil/methanol of 1/12, reaction temperature of 65oC.
KEYWORDS:Natural Ca(OH)2/KF; Superbase; Biodiesel; Palm oil
Download this article as:| Copy the following to cite this article: Nurcahyo I. F, Wijaya K, Triyono T, Budiman A, Taufiq-Yap Y. H. Synthesis and Characterization of Natural Ca(OH)2/KF Superbase Catalyst for Biodiesel Production from Palm Oil. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Nurcahyo I. F, Wijaya K, Triyono T, Budiman A, Taufiq-Yap Y. H. Synthesis and Characterization of Natural Ca(OH)2/KF Superbase Catalyst for Biodiesel Production from Palm Oil. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44409 |
Introduction
Among the studied heterogeneous catalysts, there was alkaline earth basic oxide such as CaO, MgO, BaO, and SrO. The alkaline earth basic oxide was fascinating to examine, as they have high activity to produce biodiesel1-3. The relative order of effectiveness to catalyze transesterification was BaO ~ SrO > NaOH >> CaO ~ MgO. The SrO catalyst was further examined due to toxicity concerning with BaO4. Calcium-based heterogeneous catalysts were very intriguing to develop. It is due to natural calcium abounds in the CaCO3 phase. The CaCO3 can be converted to CaO and Ca(OH)2. Their catalyst activity can be enhanced by loading potassium fluoride. The loading will produce superbase catalyst KCaF35-11. Some data concerning a state of transesterification and catalyst preparation for various catalysts of CaO and Ca(OH)2 loaded KF are shown on Table 1.
Table 1: Literature of transesterification on CaO/KF
|
Catalyst |
CaO to KF or Ca(OH)2 to KF (mol/mol) |
Calcinations |
Transesterification |
|||||
|
T (oC) |
t (h) |
Catalyst weight (%) |
Oil to Methanol (mol/mol) |
T (oC) |
t(min) |
Methyl ester yield (%) |
||
| KF/CaO5 |
4.14/1 |
600 |
4 |
4 |
1/12 |
65 |
150 |
96.8 |
| CaO/KF11 |
1/1.036 |
500 |
5 |
2.1 |
1/12 |
65 |
20 |
99.9 |
| ZnO/Ca(OH)2/KF9 |
1/1 |
400 |
6 |
3 |
1/12 |
65 |
90 |
97.6 |
| Na2SiO3/CaO/KF10 |
1/1.5 |
400 |
4 |
3 |
1/10 |
65 |
30 |
97.1 |
The data in Table 1 indicate that the CaO/KF is a good catalyst for transesterification. Both molar ratio of CaO/KF and calcinations temperature have an effect on this catalyst activity. The best of the molar ratio of CaO/KF is around 1-1.5. On the best molar ratio, transesterification of triglyceride was almost complete. The good calcinations temperature in order to prepare this catalyst is about 400-500oC. Catalyst of CaO/KF without other oxides has higher activity than the catalyst plus other oxides. The catalysts were calcined using normal heating about 400-600oC for 4-6 hours. The calcination was just wasteful of energy. In order to save energy, calcination with other methods shall be attempted. One such method is rapid thermal annealing (RTA). This method allows calcination process performed in a short time and rapid heating rate. The RTA method for calcination is superior to the CFA (conventional furnace annealing) method for reducing calcination timing budget and yielding relatively high specific surface areas of tin oxide powders12. Calcination using the RTA method produced a smaller crystalline size of the oxide than those produced using the CFA method at the same temperature12.
In this study, Ca(OH)2 was obtained from natural CaCO3 in Indonesia. The authors chose Ca(OH)2 and did not opt for CaO as its precursor. This selection is based on consideration that CaO/KF is prepared by adding water. If CaO was added by water, it will produce Ca(OH)2. The possibility of using Ca(OH)2 was more effective than CaO in formation of KCaF3. The Ca(OH)2/KF catalyst was prepared by simple grinding, thus it was calcined with Rapid Thermal Annealing method. The catalyst was characterized by X–ray diffraction (XRD), FTIR, CO2-TPD, TGA, and Surface Area Analyzer. It is applied to transesterification of palm oil and the resulting biodiesel was detected by H-NMR.
Materials and Methods
Natural Ca(OH)2 was obtained from home industry in Klaten, Indonesia. Reagent grade potassium flouride and methanol were purchased from Merck Chemical. Sample of palm oil was purchased from local store on Indonesia.
Preparation and Characterization of Ca(OH)2/KF
Amount of natural Ca(OH)2 mixed KF with molar ratio of Ca(OH)2 to KF was 0.8:1. The mixture was ground by mortar until evenly for 15 minutes. The homogenous mixture was added by 3 mL of distilled water then it was pulverized by mortar for 15 minutes. The resulted sludge was dried up at 120 oC for 6 hours. The dried sludge were crushed and filtered with 100-mesh sieve. The amount of 7 g powder was calcined at 400, 500, and 600 oC for 10 minutes by heating rate of 200oC/minutes on rapid thermal annealing (RTA) furnace (Fig 1
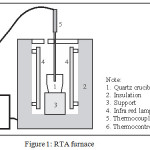 |
Figure 1: RTA furnace |
Powder XRD patterns were recorded on a Shimadzu XRD 6000 diffractometer using Cu Kα radiation (λ= 1.5418 A) at 40 kV and 30 mA. An FTIR spectrum of the catalyst was detected by using Shimadzu IR Prestige21 spectrophotometer. Basic strength of catalyst was detected by CO2-TPD on Thermo Scientific TPDRO 1100. Thermal decomposition of catalyst was investigated by TGA on Linseis STA PT1600. The surface area was detected by Quantachrome Nova 1200. The best character was chosen for variation of Ca(OH)2 to KF mol ratio. The variations were 0.8:0.5, 0.8:1, and 0.8:1.5. They were characterized by XRD, FTIR, CO2-TPD, TGA, and surface area analyzes. The obtained catalyst is abbreviated in Table 2.
Table 2: Abbreviation of catalyst
|
Mol Ca(OH)2 |
Mol KF |
Calcination temperature |
Abbreviation |
|
0.8 |
0.5 |
500 |
CaK-A-500 |
|
0.8 |
1 |
500 |
CaK-B-500 |
|
0.8 |
1.5 |
500 |
CaK-C-500 |
|
0.8 |
1 |
400 |
CaK-B-400 |
|
0.8 |
1 |
600 |
CaK-B-600 |
Transesterification of Palm oil
The transesterifications were conducted at 65oC. Experiments were carried out in a 50 mL of three-necked flasks (Fig 2). Catalysts and methanol were mixed in advance at room temperature for 15 minutes. Then the mixture was heated at 65oC. In a separate container, palm oil was heated at temperature of 65oC. This reaction set up by mixing all the reactants and catalysts at 65oC. The molar ratio of palm oil to methanol was maintained at 1:12.The catalyst used was 5 % by weight of oil. After each reaction, the catalyst was separated by using a centrifuge at 15,000 rpm for 30 second. The methyl esters were investigated at various reaction times. The investigation used Agilent of 400 MHz H-NMR Spectrometer. Conversion of palm oil to biodiesel can be measured based on Knothe’s method13 from integration values of glyceridic and methyl ester protons in H-NMR.
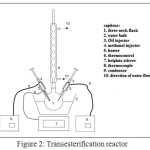 |
Figure 2: Transesterification reactor
|
Results and Discussion
Characterization of Catalyst
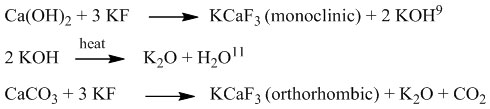
X-ray Diffraction, Fig. 3 depicts the profiles of natural Ca(OH)2 and dried natural Ca(OH)2-KF mixture. Fig. 3a represents natural Ca(OH)2 containing CaCO3 impurities. Concentration of Ca(OH)2 and CaCO3 in natural Ca(OH)2 will be discussed on TGA session. The XRD profile of the dried Ca(OH)2-KF mixture is shown in Fig. 3b. This reflects that KCaF3 has been formed on this stage in small quantities. The KCaF3 formed in 2 phases was monoclinic and orthorhombic. KCaF3 crystals are very clear when the mixture is calcined. At calcination of 500 oC, CaK-A-500 and CaK-C-500 indicates an imperfect reaction (Fig. 4). On the XRD profile, the peaks of Ca(OH)2 and CaCO3 apparently remains to have high intensity. The imperfection of reaction on CaK-A-500 was caused by deficiency of K atoms. Another case in CaK-C-500, the possibility of this imperfection due to hygroscopic catalyst was fairly high. The best catalyst was CaK-B-500 in its XRD profile, the peaks of Ca(OH)2 and CaCO3 were low, but the one with KCaF3was elevated. The monoclinic KCaF3 peaks appear at 2θ 20.08, 28.6, 40.93, 51.01, and 59.54 degrees14. The orthorhombic KCaF3 peaks come out at 2θ 35.54, 37.17, 48.10, and 68.23 degrees15. KCaF3 monoclinic formation was also reported by Fan9 in a conventionally calcined Ca(OH)2-KF reaction. Furthermore, the CaK-B-500 catalyst was selected for variations of calcination temperature. Profile of XRD in Fig. 5 indicates that monoclinic and orthorhombic KCaF3 peaks turn up. The higher the calcination temperature was, the peak intensity of Ca(OH)2 d101 (2θ 33.8 deg) was dropped off. A decrease of intensity also occurred in CaCO3. It can be confirmed by TGA (Fig. 8) and FTIR (Fig. 6). This phenomenon proves that KF is not only reactive to Ca(OH)2 but KF is also reactive to CaCO3. Thus this is the first paper postulated that KF can be reactive to CaCO3. Reaction between KF and CaCO3 produces orthorhombic KCaF3. The equation of the reaction is written as follows:
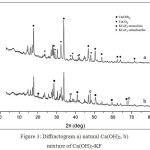 |
Figure 3: Diffractogram a) natural Ca(OH)2, b) mixture of Ca(OH)2-KF |
 |
Figure 4: XRD profile of catalysts at various molar ratios of Ca(OH)2 to KF |
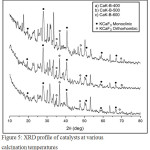 |
Figure 5: XRD profile of catalysts at various calcination temperatures
|
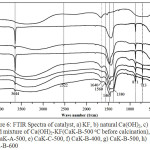 |
Figure 6: FTIR Spectra of catalyst, a) KF, b) natural Ca(OH)2, c) dried mixture of Ca(OH)2-KF(CaK-B-500oC before calcination), d) CaK-A-500, e) CaK-C-500, f) CaK-B-400, g) CaK-B-500, h) CaK-B-600 |
Fourier Transform Infrared (FTIR)
Fig. 6 depicted FTIR spectra for KF, natural Ca(OH)2, dried natural Ca(OH)2-KF mixture, and catalysts after calcinations. Fig. 6a is a spectrum of KF. The KF spectrum is similar to pure water spectrum. It indicates that KF does not absorb IR rays and KF is highly hygroscopic. On Fig. 6a-6h, broadband at 2600-3600 cm-1 is vibration of O-H in water and 1640 cm-1 corresponding to bending mode of H2O16. Vibration band at 3644 cm-1 was corresponded to O-H bond of Ca(OH)217. Peak height at 3644 cm-1 was corresponded to Ca(OH)2 concentration. The study of Ca(OH)2 concentration will be discussed at TGA session. The vibration band 1465, 1389, 873, 713 cm-1 correspond to vibration modes on CaCO317-19.
Surface area and CO2-TPD, Based on the data from nitrogen adsorption isotherms (Table 3), the catalysts were micro-porous material. The best of surface area catalyst was CaK-B-500 with surface area of 21.81 m2/g and pore diameter 1.18 nm. Character of CO2 desorption for all synthesized catalyst have superbase sites. On the data CO2-TPD (Fig. 7), CO2 desorption was occurred on 550 – 950oC. The order of basic strength is CaK-B-500 > CaK-B-600 = CaK-B-400 > CaK-C-500 > CaK-A-500. The CaK-B-500 is the best basic strength. It has two CO2-TPD peaks on 900 and 950oC. Having compared with KF/CaO that synthesized by Lin20, the CaK-B-500 seems more superior. Basic strength of KF/CaO that synthesized by Lin was 600-700oC. The high of the catalyst basic strength is derived from KCaF35 and K2O21 compounds. At Table 3, the catalyst has almost the same base. Therefore, the basic strength is very influential on catalytic ability. In this case, the CO2 detected by the TPD instrument comes from the base sites and decomposition of CaCO3, but desorption of CO2 from the decomposition was very small.
Table 3: Absorption isotherm and basicity of the catalyst
|
Catalyst |
Surface area (m2/g) |
Pore diameter (nm) |
CO2 from decomposition CaCO3 (mmol /g)a |
Basic strength (mmol/g)b |
| Natural Ca(OH)2 |
1.86 |
Not investigated |
||
| CaK-A-500 |
15.19 |
1.19 |
0.56 |
2285.18 |
| CaK-B-500 |
21.81 |
1.18 |
0.19 |
2125.77 |
| CaK-C-500 |
16.79 |
1.19 |
0.02 |
2381.20 |
| CaK-B-400 |
12.48 |
1.19 |
0.18 |
2095.40 |
| CaK-B-600 |
14.88 |
1.19 |
undetected |
1902.47 |
aData from TGA
bData from CO2-TPD
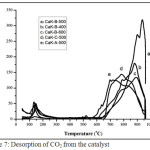 |
Figure 7: Desorption of CO2 from the catalyst Click here to View figure |
Thermogravimetry Analysis
Based on XRD data, the natural Ca(OH)2 contains Ca(OH)2 and CaCO3. Based on XRD data, natural Ca(OH)2 contains Ca(OH)2 and CaCO3. The Ca(OH)2 and CaCO3 concentrations can be measured based on TGA data. Fig 8, weight loss at temperature of 380-500oC indicates decomposition of Ca(OH)2 to CaO and H2O. The weight loss at 590-750oC represents decomposition of CaCO3 to CaO and CO2. The decomposition of Ca(OH)2 and CaCO3 at this approaching temperature were also reported in other paper22-26. Concentration of Ca(OH)2 and CaCO3 were represented on Fig. 9 and Fig.10. In Fig. 9, the higher the calcination temperature, the concentration of Ca(OH)2 and CaCO3 dropped off. This fact indicates that the higher temperature leads to an increase in reaction rate. The decrease of Ca(OH)2 and CaCO3 proves that both can be reactive to KF. In Fig. 10, an increase in KF concentration results in a decrease in Ca(OH)2 and CaCO3 concentrations. It means that an increase in concentration of the reactants will speed up the reaction rate. This phenomenon fits the law of the reaction rate. These data fit with XRD and FTIR data.
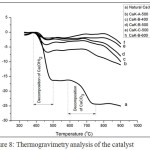 |
Figure 8: Thermogravimetry analysis of the catalyst |
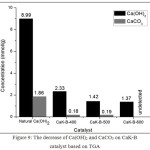 |
Figure 9: The decrease of Ca(OH)2 and CaCO3 on CaK-B catalyst based on TGA Click here to View figure |
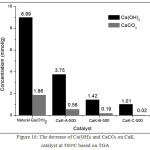 |
Figure 10: The decrease of Ca(OH)2 and CaCO3 on CaK catalyst at 500oC based on TGA Click here to View figure |
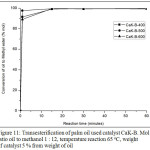 |
Figure 11: Transesterification of palm oil used catalyst CaK-B. Mol ratio oil to methanol 1:12, temperature reaction 65oC, weight of catalyst 5 % from weight of oil |
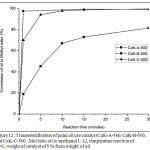 |
Figure 12: Transesterification of palm oil use catalyst CaK-A-500, CaK-B-500, and CaK-C-500 . Mol ratio oil to methanol 1: 12, temperature reaction of 65oC, weight of catalyst of 5 % from weight of oil Click here to View figure |
Transesterification of palm oil
Fig. 11, palm oil has been widely converted in 1 minute. The activity of catalyst CaK-B-500 is better than the one with CaK-B-400 and CaK-B-600 catalyst. In 1 minute, CaK-B-500 catalyst can convert palm oil to methyl ester by 97.6 %. In Fig. 12, activity of CaK-B-500 catalyst is better than the one with CaK-A-500 and CaK-C-500 catalyst. This phenomenon suits perfectly with the data of the surface area and basic strength. The CaK-B-500 catalyst has the best surface area and strength characteristics. When CaK-B-500 catalysts are compared with a homogeneous catalyst for transesterification27-29, the CaK-B-500 catalyst activity is superior. So the catalyst is potential to be used in the flow reactor.
Conclusion
A natural Ca(OH)2 containing CaCO3 impurities can be reactive to KF in order to create monoclinic and orthorhombic KCaF3. The Monoclinic KCaF3 is formed from reaction between Ca(OH)2 and KF. The orthorhombic KCaF3 is formed from the reaction between CaCO3 and KF. The natural Ca(OH)2/KF catalyst can be used as an effective catalyst for transesterification of soybean oil and methanol. Under optimum conditions, natural Ca(OH)2/KF catalyst can convert 97.6 % of palm oil into biodiesel.
Acknowledgements
The authors wish to thank Sebelas Maret University for the financial support for this work under the Grant of University Research Committee (“Hibah Penelitian Disertasi dan Doktor Baru” PNBP UNS, 2016).
References
- Su, J.; Li, Y.; Wang, H.; Yan, X.; Pan, D.; Fan, B.; Li, R., Chem. Phys. Lett., 2016, 663, 61–65
CrossRef - Bankovi, I. B.; Miladinovi, M. R.; Stamenkovi, O. S.; Veljkovi, V. B., J. Renew. Sustain. Energy, 2017, 72, 746–760
CrossRef - Teixeira, A. P. C.; Santos, E. M.; Vieira, A. F. P.; Lago, R. M., Chem. Eng. J., 2013, 232, 104–110
CrossRef - Gotcht, A. J.; Reeder, A. J; Mccormick, A., J. Undergrad. Chem. Res., 2009, 8, 22–26
- Wen, L.; Wang, Y.; Lu, D.; Hu, S.; Han, H; Fuel, 2010, 89, 2267–2271
CrossRef - Hu, S.; Wen, L.; Wang, Y.; Zheng, X.; Han, H, Bioresour. Technol., 2012, 123, 413–418
CrossRef - Fan, M.; Zhang, P.; Ma, Q, Bioresour. Technol., 2012, 104, 447–450
CrossRef - Guo, F.; Peng, Z. G.; Dai, J. Y.; Xiu, Z. L., Fuel Process. Technol., 2010, 91, 322–328
CrossRef - Fan, F.; Gao, C.; Jia, L.; Guo, X., Res. Chem. Intermed., 2014, 40, 157–167
CrossRef - Fan, F.; Gao, C.; Jia, L.; Guo, X., Asian J. Chem., 2013, 25, 4705–4706
CrossRef - Liu, H.; Su, L.; Shao, Y.; Zou, L., Fuel, 2012, 97, 651–657
CrossRef - Son, H. H.; Lee, W. G., J. Ind. Eng. Chem., 2012, 18, 317–320
CrossRef - Knothe, G., J. Am. Oil Chem. Soc., 2000, 77, 489–493
CrossRef - Ratuszna, A.; Rousseau, M.; Daniel, P., Powder Diffr., 1997, 12, 70–75
CrossRef - Knight, K. S.; Darlington, C. N. W.; Wood, I. G., Powder Diffr., 2005, 20, 7–13
CrossRef - Lou, S.; Jia, L.; Guo, X.; Wu, P.; Gao, L.; Wang, J., SpringerPlus, 2015, 4, 686
CrossRef - Galván-Ruiz, M.; Hernández, J.; Baños, L.; Noriega-Montes J.; Rodríguez-García, M. E., J. Mater. Civ. Eng., 2009, 21, 694–69
CrossRef - Lesbani, A.; Tamba, P.; Mohadi, R.; Fahmariyanti., Indones. J. Chem., 2013, 13, 176–180
CrossRef - Zeng, H.;Feng, Z.; Deng, X.; Li, Y., Fuel, 2008, 87, 3071–3076
CrossRef - Lin, L.; Cui, H.; Vittayapadung, S.; Xiao, Z.; Zhang, A.; Liu, R.; and Li, C., Eur. J. Lipid Sci. Technol., 2015, 117, 406–410
CrossRef - Sibarani, J.; Khairi, S.; Wijaya, K.; Tahir, I., Indones. J. Chem., 2007, 7, 314–319
- Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E., J. Ind. Eng. Chem., 2014, 20, 113–117
CrossRef - Georgieva, V.; Vlaev, L.; Gyurova, K.; J. Chem., 2013, 2013, 1-12
- Popescu, M. A.; Isopescu, R.; Matei, C.; Fagarasan, G.; Plesu, V., Adv. Powder Technol. 2014, 25, 500–507
CrossRef - Li, Z.; Huang, C.; Guo, L.; Cui, L.; Zhou, B., Colloids Surf A Physicochem Eng Asp., 2016, 498, 98–105
CrossRef - Fauziah, T. R.; Sangi, M. S; Oetami, T. P., Indones. J. Chem., 2016, 16, 208–213
- Kawentar, W. A.; Budiman, Energy Procedia, 2013, 32, 190–199
CrossRef - Darnoko, D.; Cheryan, M., JAOCS, 2000, 77, 1263–1267
CrossRef - Yoeswono; Triyono; Tahir, I., Indones. J. Chem., 2008, 8, 219–225

This work is licensed under a Creative Commons Attribution 4.0 International License.









