Synthesis and Biological Evaluation of Certain new Cyclohexane-1-carboxamides as Apoptosis Inducers
Walaa Hamada Abd-Allah1 and Mohamed Fathy Elshafie2
1Pharmaceutical Chemistry Department, Collage of Pharmaceutical Science and Drug Manufacturing, Misr University for Scienceand Technology, P.O. 77, 6th of October City, Giza, Egypt.
2Pharmacology, Toxicology Department, Faculty of Pharmacy, Al-Azhar University, Nasr City Cairo, Egypt.
Corresponding Author E-mail : Walaa.abdalla@must.edu.eg
DOI : http://dx.doi.org/10.13005/ojc/340228
Article Received on : January 20, 2018
Article Accepted on : March 28, 2018
Series of 1-(N-phenyl-2-(heteroalicyclic-1-yl)acetamido)cyclohexane-1-carboxamide derivatives (5a-m) and 1-(phenyl(heteroalicyclic-1-ylmethyl)amino)cyclohexane-1-carboxamide (6a-f) were designed and synthesized with biological interest through coupling of 1-(2-chloro-N-phenylacetamido)cyclohexane-1-carboxamide (4) and (phenylamino)cycloakanecarboxamide (2) with different amines. The structures of the target compounds were elucidated via IR, 1H and 13C NMR, MS, and microanalysis. Compounds 5a-m and 6a-f were evaluated for their in-vitro antitumor activity against four different cancer cell lines, MCF-7, HepG2, A549, and Caco-2. Compound 5i exhibited a promising activity against breast cancer cell line (IC50 value = 3.25 μM) compared with doxorubicin (IC50 value = 6.77 μM). Results from apoptosis and cell cycle analysis for compound 5i revealed good antitumor activity against MCF-7 cancer cell line and potent inhibition.
KEYWORDS:1,1-Disubstituted cyclohexane; Amides; Synthesis; Cytotoxic evaluation; Apoptosis
Download this article as:| Copy the following to cite this article: Abd-Allah W. H, Elshafie M. F. Synthesis and Biological Evaluation of Certain new Cyclohexane-1-carboxamides as Apoptosis Inducers. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Abd-Allah W. H, Elshafie M. F. Synthesis and Biological Evaluation of Certain new Cyclohexane-1-carboxamides as Apoptosis Inducers. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44707 |
Introduction
Cancer is one of the most prominent diseases worldwide which represents the second cause of human mortality after cardiovascular diseases1. Drug resistance to cancer chemotherapy is considered a serious trouble2. Thus, there is a critical need for new chemotherapeutic agents3,4. The cyclohexane core generally has enriched the medicinal chemistry armamentarium with several bioactive candidates having diverse biological activities such as antipsychotic5, expectorant6, anticonvulsant7,8, analgesic9 and anticancer activities10-12. Etoposide (I) and Teniposide (II) contain cyclohexane moiety in their structures and are used in cancer chemotherapy for the treatment of lung cancer, acute leukemia and lymphoma through a cytotoxic mechanism of DNA- topoisomerase II inhibition13-15. Moreover, the aminoacyl pharmacophore chain and amide moiety were included in the structural frame of different antitumor compounds, III and IV (Fig. 1), which were found to possess antitumor activity16-19.
These findings have encouraged us to prepare the target compounds 5a-m and 6a-f through molecular hybridization tactic of two or more pharmacophore moieties in one molecule aiming to improve the pharmacological profile20.
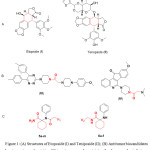 |
Figure 1: (A) Structures of Etoposide (I) and Teniposide (II); (B) Antitumor biocandidates bearing aminoacyl moieties; (C) target compounds containing the pharmacophoric features. |
Results and Discussion
Chemistry
The preparation of the ultimate compounds 5a–m and 6a–f as well as the intermediates 1–4 is illustrated in Scheme 1. Cyclohexanone was reacted with potassium cyanide and aniline in glacial acetic acid to produce the nitrile derivative 1 which was hydrolyzed using sulfuric acid at room temperature to produce the amidic compound 3.
The penultimate intermediate 4 was achieved by acetylation of compound 3. Subsequently, compound 4 was subjected to the reaction with different amines to afford 5a-m, while compound 3 was reacted with formaldehyde and different amines to afford 6a-f.
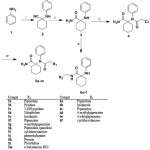 |
Scheme 1 |
Reagents and conditions: i) Cyclohexanone, potassium cyanide, acetic acid glacial, r.t, 24 h ii) H2SO4, r.t, 48 h, iii) ClCOCH2Cl, CHCl3, r.t , 24 h, iv) ethanol, appropriate amine, 12 h, reflux v) HCHO, ethanol, appropriate amine, 12 h, reflux.
Biological Evaluation
Antiproliferation Assay
The antitumor activity for compounds 5a-m and 6a-f were evaluated against the four cancer cell lines HepG2 (liver), MCF-7 (breast), A549 (lung) and Caco-2 (colorectal). Results are illustrated in (Table 1). Most of the compounds are selective and having potential cytotoxicity towards MCF-7 adenocarcinoma with IC50 values range 3.25-36.8 μM as compared with doxorubicin (IC50 value of 6.77 μM). Compound 5i showed the most potent biological activity with IC50 value = 3.25 μM.
Additionally, compound 5i showed high potential activity toward human HepG2 hepatocellular carcinoma with IC50 11.5 μM, A549 lung adenocarcinoma with IC50 6.95 μM and Caco-2 colorectal adenocarcinoma with IC50 8.98 μM as compared with doxorubicin (3.07, 0.887, 2.78 μM respectively). Examining selectivity, the test compounds showed high activity against MCF-7.
Table 1: Antiproliferative activity for compounds 5a-m and 6a- f.
| Compound |
MCF-7 IC50 μM |
HepG2 IC50 μM |
A549 IC50 μM |
Caco-2 IC50 μM |
| 5a |
26.15 |
50.2 |
35.4 |
30.21 |
| 5b |
3.68 |
10.61 |
7.98 |
5.46 |
| 5c |
7.21 |
15.02 |
9.97 |
14.12 |
| 5d |
28.41 |
60.3 |
42.7 |
52.40 |
| 5e |
31.22 |
56.4 |
40.6 |
50.22 |
| 5f |
20.08 |
43.32 |
39.74 |
4.33 |
| 5g |
9.13 |
33.88 |
30.3 |
25.43 |
| 5h |
22.68 |
58.3 |
31.29 |
47.23 |
| 5i |
3.25 |
11.5 |
6.95 |
8.98 |
| 5j |
13.59 |
31.28 |
15.64 |
22.34 |
| 5k |
5.48 |
20.71 |
14.59 |
13.66 |
| 5l | 4.58 |
12.08 |
8.51 |
11.25 |
| 5m |
6.48 |
13.82 |
11.83 |
10.53 |
| 6a | 36.8 |
– |
– |
– |
| 6b |
23.51 |
16.46 |
31.21 |
28.67 |
| 6c |
– |
– |
– |
– |
| 6d |
17.05 |
15.91 |
26.22 |
39.52 |
| 6e |
26.13 |
21.74 |
42.09 |
51.43 |
| 6f |
22.89 |
17.54 |
31.6 |
44.12 |
| Doxorubicin |
6.77 |
3.07 |
0.887 |
2.78 |
(-): No activity
Compound 5i was the most potent against MCF-7 cancer cell line. Consequently, we examined its effect on the cell cycle progression using BD FASCCalibur after treatment with 3.25 μM of 5i for 48 h. Then, cell was stained with an annexin V-FITC antibody and propidium iodide by FACS (Table 2). For the cell cycle, compound 5i revealed induction of apoptosis at pre G1 phase and arresting at G2/M phase.
Table 2: Cell cycle analysis for the control and compound 5i at concentration of 3.25 μM for 48 h on MCF-7 cell line.
| Sample data | Result | ||||
| %G0-G1 | %S | % G2-M | % Apoptosis | Comment | |
| 5i | 7.66 | 24.77 | 46.17 | 21.4 | PreG1 apoptosis &cell growth arrest@G2/M |
| Cont. MCF-7 | 71.7 | 22.45 | 5.23 | 0.62 | Control pattern |
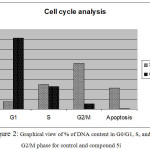 |
Figure 2: Graphical view of % of DNA content in G0/G1, S, and G2/M phase for control and compound 5i
|
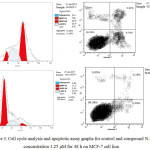 |
Figure 3: Cell cycle analysis and apoptotic assay graphs for control and compound 5i at concentration 3.25 μM for 48 h on MCF-7 cell line. |
Materials and Methods
Chemistry
General
Melting points were measured through Electrothermal Capillary apparatus and are uncorrected. The infrared (IR) spectra were recorded on JASCO FT/IR-6100 spectrometer. Spectral data (1H-NMR as well as 13C-NMR) were performed on Jeol ECA 500 MHz spectrometer and their values of the chemical shift are recorded as ppm on δ scale. Mass spectral data were gained using the technique of electron impact (EI) ionization. Column chromatography was conducted using silica gel 60 and chloroform/methanol 9/1 (v/v) as a mobile phase.
Synthesis of 1-(phenylamino)cyclohexanecarbonitrile (2)
Compound 2 was prepared as showed in literatures8.
Synthesis of (phenylamino)cycloakanecarboxamide (3)
Compound 3 was prepared as showed in literatures8.
Synthesis of 1-(2-chloro-N-phenylacetamido)cyclohexane-1-carboxamide (4)
Chloroacetyl chloride (1.317g, 0.95 mL, 0.01166 mol) was added to a solution of 3 (2.1g, 0.0097 mol) in chloroform (30 mL). The mixture was allowed for stirring at room temperature for 24h, then adding an aqueous solution of 10% NaOH (2 x 30 mL) then separation of the organic layer, drying over anhydrous Na2SO4 and evaporation under reduced pressure to afford 2.5 g of 4 as pale yellow oil in 89.2% yield which is solidified upon standing at room temperature, m.p.170 33/°C. IR (KBr, cm-1) 2926.23, 2851.17 (NH2), 1645, 1625 (2 x C=O) ; 1H NMR (CDCl3) 1.34-1.52 (m, 10H, 5 x CH2, cyclohexyl), 3.26 (s, 2H, CH2-Cl), 6.99 (s, 2H,NH2), 7.25-7.32 (m, 5H, Har.); 13C NMR (CDCl3) δ ppm 20.79, 22.91, 32.82 (3 x CH2, cyclohexyl), 40.41 (CH2-Cl), 66.60 (Cq), 128.60, 128.77, 130.23 (3CHar.), 139.16 (Car.), 167.16 (CO-CH2), 178.60 (CO-NH2); MS (EI) m/z (%): 294.78 ([M]+, 1.60, ), 250 (100). (EI) m/z (%): 294.78 ([M]+, 5), 250 (100). Anal. calcd. for C15H19ClN2O2: C,61.12; H,6.50; Cl, 12.03; N, 9.50. Found: C, 61.23; H, 6.51; Cl, 12.23; N, 9.49.
General procedure for the synthesis of 1-(N-phenyl-2-(heteroalicyclic-1-yl)acetamido)- cyclohexane-1-carboxamides (5a-m)
To a solution of 4 (1.32 g, 0.0045 mol) in ethanol (30 mL), the appropriate amine derivative (0.0135 mol) was added. Then the reaction was refluxed under stirring for 12 h, and then ethanol was evaporated under reduced pressure. The residual was dissolved in ethyl acetate (30 mL) and washed with water (3×30 mL) then separation of the organic layer, drying over anhydrous Na2SO4 and evaporation under the reduced pressure to produce 5a-m.
1-(N-Phenyl-2-(piperidin-1-yl)acetamido)cyclohexane-1-carboxamide (5a)
White solid, m.p. 120 °C, yield 75%; IR (KBr, cm-1) 2924, 2841 (NH2), 1635, 1621 (2 x C=O); 1H NMR: 1.12 (s, 10H, 5 x CH2, cyclohexyl), 1.78 (s, 10H, 5 x CH2, piperidine), 3.16 (s, 2H, CO-CH2), 6.53 (s, 2H, NH2), 7.18-7.53 (m, 5H, Har.); 13C NMR: 20.96, 22.34, 22.87 (3 x CH2, cyclohexyl), 25.08, 25.66 (2 x CH2-piperidine), 58.81 (CH2-piperidine), 54.44 (CO-CH2), 118.48, 128.85, 129.67 (3 x CHar.), 144.17 (Car.), 168.55 (CO-CH2), 179.90 (CO-NH2); MS (EI) m/z (%): 343.47 ([M]+, 1.83), 47.9 (100). Anal. calcd. for C20H29N3O2: C, 69.94; H, 8.51; N,
12.23; O, 9.32. Found: C, 69.74; H, 8.72; N, 12.41; O, 9.33.
1-(N-Phenyl-2-(pyridin-1(2H)-yl)acetamido)cyclohexane-1-carboxamide (5b)
Yellow viscous oil, yield 85%, IR (KBr, cm-1) 2935.13, 2860.88 (NH2), 1742.37, 1660.41 (2 x C=O); 1H NMR: 1.14-1.35 (s, 10H, 5 x CH2, cyclohexyl), 3.62 (s, 2H, CH2-pyridine), 3.64 (s, 2H, CO-CH2), 6.03 (s, 2H, NH2), 6.63 (d, 1H, J = 7.6 Hz, CH-pyridine.), 6.82 (d, 1H, J = 7.6 Hz, CH-pyridine), 6.91 (d, 1H, J = 7.6 Hz, CH-pyridine), 7.10 (s, 1H, CH-pyridine), 7.37-7.91 (m, 5H, Har.); 13C NMR: 21.07, 22.20, 25.11 (3 x CH2, cyclohexyl), 52.21 (CH2-pyridine), 60.22 (CO-CH2), 64.43 (Cq), 118.98, 125.94, 131.54, (3 x CHar.), 143.86 (Car.), 167.79 (CO-CH2), 179.66 (CO-NH2), MS (EI) m/z (%): 339.41 ([M]+, 1.19), 174 (100). Anal. calcd. for C20H25N3O2: C, 70.77; H, 7.42; N, 12.38; O, 9.43. Found: C, 70.57; H, 7.44; N, 12.33.
1-(2-(3-Hydroxypyridin-1(2H)-yl)-N-phenylacetamido)cyclohexane-1-carboxamide (5c)
Yellow viscous oil, yield 90%, m.p. 92 °C, IR (KBr, cm-1) 2932, 2859 (NH2), 1718, 1655 (2 x C=O); 1H NMR: 1.34-2.19 (m, 10H, 5 x CH2, cyclohexyl), 3.71 (s, 2H, CH2-pyridine), 3.92 (s, 2H, CO-CH2), 6.65 (s, 2H, NH2), 6.67 (s, 1H, CH-pyridine), 7.13 (s, 1H, CH-pyridine), 7.49 (s, 1H, CH-pyridine), 8.06 (s, 3H, Har.), 8.33 (s, 2H, Har.), 10.04 (br. s, 1H, OH); 13C NMR: 22.53, 25.73, 33.21 (3 x CH2, cyclohexyl), 48.91 (CH2-pyridine), 55.34 (CO-CH2), 68.71 (Cq), 94.23 (CH-pyridine), 116.12, 125.63, 127.11 (3 x CHar.), 134.71 (Car.), 136.44 (CH-N), 156.11 (C-OH), 167.21 (CO-CH2), 181.42 (CO-NH2); (EI) m/z (%): 355.44 ([M]+, 2.5), 336 (100). Anal. calcd. for C20H25N3O3: C, 67.58; H, 7.09; N, 11.82; O, 13.50. Found: C, 67.48; H, 7.12; N, 11.71.
1-(2-Morpholino-N-phenylacetamido)cyclohexane-1-carboxamide (5d)
Pale yellow oil, yield 90%, IR (KBr, cm-1) 2906, 2860 (NH2), 1716, 1658 (2 x C=O); 1H NMR: 1.51-2.09 (m, 10H, 5 x CH2, cyclohexyl), 2.51 (t, 2H, J = 6 Hz, CH2-morpholine), 3.71 (t, 2H, J = 6 Hz, CH2-morpholine), 3.88 (s, 2H, CO-CH2), 6.93 (s, 2H, NH2), 7.15-7.19 (m, 3H, Har.), 7.33 (s, 2H, Har.); (EI) m/z (%): 345.44 ([M]+,0.81), 174 (100). Anal. calcd. for C19H27N3O3: C, 66.06; H, 7.88; N, 12.16. Found: C, 66.21; H, 7.75; N, 12.24.
1-(2-(1H-Imidazol-1-yl)-N-phenylacetamido)cyclohexane-1-carboxamide (5e)
Yellow viscous oil, yield 90%, IR (KBr, cm-1) 2933, 2852 (NH2), 1739, 1669 (2 x C=O); 1H NMR: 1.17-1.54 (s, 10H, 5 x CH2, cyclohexyl), 3.63 (s, 2H, CO-CH2), 6.61 (s, 2H, NH2), 7.03 (s, 3H, CH-imidazole), 7.67 (s, 5H, Har.); 13C NMR: 20.97, 25.12, 31.24 (3 x CH2, cyclohexyl), 53.12 (CO-CH2), 59.99 (Cq), 115.19, 115.98, 121.51, 129.02, 129.24, 130.13 (3 x CH-imidazole, 3 x CHar.), 144.17 (Car.), 168.21 (CO-CH2), 180.13 (CO-NH2), (EI) m/z (%): 326.40 ([M]+, 15), 174 (100). Anal. calcd. for C18H22N4O2: C, 66.24; H, 7.79; N, 17.17. Found: C, 66.21; H, 7.81; N, 17.25.
1-(N-Phenyl-2-(piperazin-1-yl)acetamido)cyclohexane-1-carboxamide (5f)
Brown viscous oil, yield 79%, IR (KBr, cm-1) 2935, 2857 (NH2), 1739.48, 1649.81 (2 x C=O); 1H NMR: 1.24-1.60 (m, 11H, 5 x CH2, cyclohexyl, NH), 1.96 (s, 4H, 2 x CH2-piperazine), 2.07 (s, 4H, 2 x CH2-piperazine), 3.02 (s, 2H, CO-CH2), 6.93 (s, 2H, NH2), 7.15-7.42 (m, 5H, Har.); 13C NMR: 21.04, 25.13, 31.20 (3 x CH2, cyclohexyl), 41.96, 44.45 (2 x CH2-piperazine), 59.99 (CO-CH2), 60.38 (Cq), 116.04, 118.85, 129.01 (3 x CHar.), 144.15 (Car.), 171.15 (CO-CH2), 179.61 (CO-NH2), (EI) m/z (%): 344.46 ([M]+, 3.03), 174 (100). Anal. calcd. for C19H28N4O2: C, 66.25; H, 8.19; N, 16.27. Found: C, 66.33; H, 8.22; N, 16.22.
1-(2-(4-Methylpiperazin-1-yl)-N-phenylacetamido)cyclohexane-1-carboxamide (5g)
Yellow viscous oil, yield 75%, IR (KBr, cm-1) 2935, 2855 (NH2), 1739, 1668 (2 x C=O); 1H NMR: 1.25-1.61 (m, 10H, 5 x CH2, cyclohexyl), 1.97 (s, 3H, CH3), 2.29 (s, 4H, 2 x CH2-piperazine), 2.68 (s, 4H, 2 x CH2-piperazine), 3.21( s, 2H, CH2-Me-piperazine), (s, 2H, CO-CH2), 6.81 (s, 2H, NH2), 6.91-7.52 (m, 5H, Har.); 13C NMR: 21.05, 25.12, 31.19 (3 x CH2, cyclohexyl), 45.75 (CH3), 52.70, 54.52 (2 x CH2-piperazine), 54.65 (CO-CH2), 66.02 (Cq), 118.90, 129.02, 130.71 (3 x CHar.), 144.12 (Car.), 169.51 (CO-CH2), 179.57 (CO-NH2), (EI) m/z (%): 358.49 ([M]+, 1.8), 113 (100). Anal. calcd. for C20H30N4O2: C, 67.01; H, 8.44; N, 15.63. Found: C, 67.22; H, 8.52; N, 15.73.
1-(2-(4-(7-Chloroquinolin-4-yl)piperazin-1-yl)-N-phenylacetamido)cyclohexane-1-carboxamide (5h)
Brown viscous oil, yield 75%, IR (KBr, cm-1) 2935, 2860 (NH2), 1739, 1654 (2 x C=O); 1H NMR: 1.26-1.49 (m, 10H, 5 x CH2, cyclohexyl), 2.18 (s, 4H, 2 x CH2-piperazine), 3.64 (s, 4H, 2 x CH2-piperazine), 3.81 (s, 2H, CO-CH2), 6.89 (br. s, 2H, NH2), 7.14 (d, 1H, J = 6.75 Hz, CH quinoline), 7.19 (d, 1H, J = 7.5 Hz, CH quinoline), 7.22 (s, 5H, Har.), 7.35 (s, 2H, CH quinoline), 7.46 (d, 1H, J = 5, CH quinoline); 13C NMR: 22.35, 25.10, 33.58 (3 x CH2, cyclohexyl), 51.21, 54.32 (2 x CH2-piperazine), 56.91 (CO-CH2), 67.81 (Cq), 128.87, 129.11, 129.51, 129.61, 129.87, 130.33, 133.42, 133.63 (6 x CHar.), 135.61, 137.82, 152.81, 153.53, 154.22 (5 x Car.), 166.21 (CO-CH2), 178.93 (CO-NH2); (EI) m/z (%): 506 ([M]+, 7.2), 288 (100). Anal. calcd. for C28H32ClN5O2: C, 66.46; H, 6.37; Cl, 7.01; N, 13.84. Found: C, 66.42; H, 6.36; Cl, 7.11; N, 13.82.
1-(2-(Cyclohexylamino)-N-phenylacetamido)cyclohexane-1-carboxamide (5i)
Brown viscous oil, yield 80%, IR (KBr, cm-1) 2933, 2856 (NH2), 1745, 1647 (2 x C=O); 1H NMR: 1.02-1.18 (m, 10H, 5 x CH2, cyclohexyl), 1.45-1.85 (m, 10H, 5 x CH2, cyclohexyl), 2.78 (s, 2H, CO-CH2), 5.51 (br. s, 1H, NH), 6.56 (s, 2H, NH2); 13C NMR: 21.73, 24.53, 25.18, 29.61, 30.21, 32.40 (6 x CH2, cyclohexyl), 50.23 (CO-CH2), 59.78, 63.50 (2 x Cq), 115.04, 115.83, 129.77 (3 x CHar.), 144.19 (Car.), 162.81 (CO-CH2), 179.88 (CO-NH2), (EI) m/z (%): 357.50 ([M]+, 0.77), 174 (100). C21H31N3O2: C, 70.55; H, 6.74; N, 11.75. Found: C, 70.43; H, 6.72; N, 11.72.
1-(2-(Phenethylamino)-N-phenylacetamido)cyclohexane-1-carboxamide (5j)
Brown viscous oil, yield 85%, IR (KBr, cm-1) 2933, 2862 (NH2), 1747, 1647 (2 x C=O); 1H NMR: 1.92 (s, 10H, 5 x CH2, cyclohexyl), 2.83 (t, 2H, J = 7.45 Hz , CH2-C6H5), 2.99 (t, 2H, J = 7.25 Hz, NH-CH2-CH2), 4.43 (s, 2H, CO-CH2), 6.82 (s, 2H, NH2), 7.17-7.27 (m, 10H, Har.); 13C NMR: 22.81, 23.17, 25.16 (3 x CH2, cyclohexyl), 35.52 (CH2-C6H5), 42.46 (NH-CH2-CH2) 52.51 (CO-CH2), 73.12 (Cq), 115.15, 116.06, 126.44, 127.51, 128.58, 128.84 (6 x CHar.), 138.82, 142.51 (2 x Car.), 161.64 (CO-CH2), 181.71 (CO-NH2), (EI) m/z (%): 379.50 ([M]+, 5.29), 174 (100). C23H29N3O2: C, 72.79; H, 7.70; N, 11.07. Found: C, 72.81; H, 7.85; N, 11.15.
1-(N-Phenyl-2-(1H-pyrrol-1-yl)acetamido)cyclohexane-1-carboxamide (5k)
Brown viscous oil, yield 78%, IR (KBr, cm-1) 2935, 2862 (NH2), 1747, 1647 (2 x C=O); 1H NMR: 1.63-2.21 (m, 10H, 5 x CH2, cyclohexyl), 3.77 (s, 2H, CO-CH2), 5.61 (s, 2H, NH2), 6.63-6.80 (m, 4H, CH-pyrrole), 7.28-7.46 (m, 5H, Har.); (EI) m/z (%): 325.41 ([M]+, 3.45), 174 (100). C19H23N3O2: C, 70.13; H, 7.12; N, 12.91. Found: C, 70.25; H, 7.22; N, 12.85.
1-(N-Phenyl-2-(pyrrolidin-1-yl)acetamido)cyclohexane-1-carboxamide (5l)
Brown solid, m.p. 100 °C, yield 75%, IR (KBr, cm-1) 2933, 2858 (NH2), 1743, 1662 (2 x C=O; 1H NMR: 1.13-1.34 (m, 10H, 5 x CH2, cyclohexyl), 2.01 (s, 4H, 2 x CH2-pyrrolidine), 2.19 (s, 4H, 2 x CH2-pyrrolidine), 3.67 (s, 2H, CO-CH2), 6.84 (s, 2H, NH2), 7.20-7.47 (m, 5H, Har.); 13C NMR: 21.07, 22.41, 25.08, 33.63, 30.21 (3 x CH2, cyclohexyl, CH2-pyrrolidine), 52.32 (CH2-pyrrolidine), 56.93 (CO-CH2), 65.61 (Cq), 129.10, 129.41, 129.56 (3 x CHar.), 130.49 (Car.), 162.54 (CO-CH2), 179.81 (CO-NH2), (EI) m/z (%): 329.44 ([M]+,0.75), 174 (100). C19H27N3O2: C, 69.27; H, 8.26; N, 12.76. Found: C, 69.22; H, 8.31; N, 12.82.
1-(2-(Butylamino)-N-phenylacetamido)cyclohexane-1-carboxamide (5m)
Brown viscous oil, yield 85%, IR (KBr, cm-1) 2933, 2862 (NH2), 1747, 1658 (2 x C=O); 1H NMR: 0.891 (t, 3H, J = 7 , CH3), 1.25 (s, 2H, CH2-CH3), 1.32-1.39 (m, 10H, 5 x CH2, cyclohexyl), 2.04 (s, 2H, CH2-CH2-CH3), 2.17 (t, 2H, J = 7 Hz, NH-CH2), 2.89 (br. s, 1H, NH), 3.21 (s, 2H, CO-CH2), 6.81 (s, 2H, NH2), 7.31-7.62 (m, 5H, Har.); 13C NMR: 21.04 (CH3), 13.54, 22.47, 24.97, 29.50, 30.93 (3 x CH2, cyclohexyl, –CH2–CH2-CH3), 45.84 (NH-CH2), 51.32 (CO-CH2), 60.39 (2 x Cq), 115.12, 116.10, 129.04 (3 x CHar.), 144.07 (Car.), 167.33 (CO-CH2), 178.91 (CO-NH2), (EI) m/z (%): 367.95 ([M]+,1.68), 174 (100). C19H30ClN3O2: C, 62.03; H, 8.22; Cl, 9.64; N, 11.42. Found: C, 62.21; H, 8.32; N, 11.35.
General procedure for the synthesis of 1-(phenyl(heteroalicyclic-1-ylmethyl)amino) cyclohexane-1-carboxamides (6a-f)
To a solution of compound 3 (2.0 g, 0.01 mol) in 96% ethanol, 40% solution of formaldehyde (0.3 g, 0.01 mol) and the appropriate amine was added. The reaction mixture was refluxed for 12 h, then allowed to evaporate under reduced pressure and purified using column chromatography [chloroform (9): ethyl acetate (1)] to afford compounds 6a-f.
1-(phenylamino)-N-(piperidin-1-ylmethyl)cyclohexane-1-carboxamide (6a)
White solid, yield 90%, m.p. 110 °C, IR (KBr, cm-1) 2924, 1681 (C=O); 1H NMR: 1.27-1.71 (m, 10H, 5 x CH2, cyclohexyl), 1.74-2.07 (m, 10H, 5 x CH2, piperidine), 4.86 (s, 2H, CH2-piperidine), 6.91 (s, 3H, Har.), 7.30 (s, 2H, Har.);13C NMR: 22.12, 24.73, 29.59, (3 x CH2, cyclohexyl), 29.83, 30.51, 31.22, (3 x CH2, piperidine), 66.23 (CH2-N), 67.88 (Cq), 116.02, 119.16, 129.18 (3 x CHar.), 143.37 (Car.), 175.57 (CO-NH), (EI) m/z (%): 315.23 ([M]+, 5.3), 189 (100). C19H29N3O: C, 72.34; H, 9.27; N, 13.32. Found: C, 72.41; H, 9.25; N, 13.45.
N-((1H-Imidazol-1-yl)methyl)-1-(phenylamino)cyclohexane-1-carboxamide (6b)
Yellow viscous oil, yield 92%; IR (KBr, cm-1) 2941, 1600 (C=O); 1H NMR: 1.27-1.29 (m, 10H, 5 x CH2, cyclohexyl), 5.37 (s, 2H, CH2-Imidazole), 6.87-6.94 (m, 3H, 3 x CH-imidazole), 7.00-7.49 (m, 5H, Har.); 13C NMR: 20.68, 27.22, 31.00 (3 x CH2, cyclohexyl), 61.71 (CH2-imidazole), 66.22 (Cq), 113.32, 117.65, 118.73, 123.10, 128.37, 130.10 (3 x CHar, 3 x CH-imidazole), 145.15 (Car.), 175.23 (CO-NH), (EI) m/z (%): 298.39 ([M]+,1.38). C17H22N4O: C, 68.43; H, 7.43; N, 18.78. Found: C, 68.38; H, 7.55; N, 18.91.
1-(Phenylamino)-N-(piperazin-1-ylmethyl)cyclohexane-1-carboxamide (6c)
White solid, m.p. 130 °C, yield 80%; IR (KBr, cm-1) 2924, 1600 (C=O); 1H NMR: 1.21 (s, 1H, NH piperazine), 1.71 (t, 2H, J = 10 Hz, CH2-piperazine), 1.92-2.55 (m, 10H, 5 x CH2, cyclohexyl), 2.74 (t, 2H, J = 10 Hz, CH2-piperazine), 4.82 (s, 2H, CH2-piperazine), 6.88-7.29 (m, 5H, Har.); 13C NMR: 22.07, 25.10, 29.88 (3 x CH2, cyclohexyl), 49.13, 54.53 (2 x CH2-piperazine), 60.58 (CH2-piperazine), 62.87 (Cq), 116.15, 119.12, 129.14 (3 x CHar.), 143.40 (Car.), 175.37 (CO-NH), (EI) m/z (%): 345.44 ([M]+,0.81), (EI) m/z (%): 316.45 ([M]+, 20.23), 99 (100). C18H28N4O: C, 68.32; H, 8.92; N, 17.71. Found: C, 68.35; H, 8.85; N, 17.75.
N-((4-Methylpiperazin-1-yl)methyl)-1-(phenylamino)cyclohexane-1-carboxamide (6d)
Yellow viscous oil, yield 85%; IR (KBr, cm-1) 2921, 1621 (C=O); 1H NMR: 1.24-1.25 (m, 10H, 5 x CH2, cyclohexyl), 2.31 (s, 3H, CH3), 2.64 (s, 4H, 2 x CH2– piperazine), 3.51 (s, 4H, 2 x CH2-piperazine), 4.79 (s, 2H, CH2-Methyl piperazine), 7.02-7.22 (m, 5H, Har.); 13C NMR: 15.16, 18.46, 20.12 (3 x CH2, cyclohexyl), 45.88 (CH3), 51.72, 54.55 (2 x CH2-Me-piperazine), 62.27 (CH2-Methyl piperazine), 68.60 (Cq), 114.57, 120.92, 129.02 (3 x CHar.), 146.53 (Car.), 177.53 (CO-NH), (EI) m/z (%): 330.48 ([M]+, 11.4), 76.93 (100). C19H30N4O: C, 69.05; H, 9.15; N, 16.95. Found: C, 69.12; H, 9.23; N, 16.85.
N-((4-Ethylpiperazin-1-yl)methyl)-1-(phenylamino)cyclohexane-1-carboxamide (6e)
Brown viscous oil, yield 75%; IR (KBr, cm-1) 2935, 1621 (C=O); 1H NMR: 1.09 (t, 3H, J = 4.5 Hz, CH3), 1.11-1.24 (m, 10H, 5 x CH2, cyclohexyl), 2.32 (s, 2H, CH2-CH3), 2.47 (t, 2H, J = 8.9 Hz, CH2– piperazine), 2.66 (t, 4H, J = 5.8 Hz, CH2-piperazine), 4.79 (s, 2H, CH2-Methyl piperazine), 7.00-7.09 (m, 3H, Har.), 7.27 (s, 2H, Har); (EI) m/z (%): 344.50 ([M]+,0.75), 127 (100). C20H32N4O: C, 69.73; H, 9.36; N, 16.26. Found: C, 69.77; H, 9.42; N, 16.35.
N-((Cyclohexylamino)methyl)-1-(phenylamino)cyclohexane-1-carboxamide (6f)
Brown solid, m.p.130 °C, yield 80%; IR (KBr, cm-1) 2929, 1600 (C=O); 1H NMR: 1.25-1.77 (m, 10H, 5 x CH2, cyclohexyl), 1.78-2.94 (m, 10H, 5 x CH2, cyclohexyl), 4.64 (s, 2H, CH2-NH), 6.92-7.28 (m, 5H, Har); 13C NMR: 20.09, 25.61, 28.75, 36.87, 43.42, 48.13 (6 x CH2, 2cyclohexyl), 56.13 (CH2-NH), 58.15, 62.46 (2 x Cq), 113.08, 128.97, 129.49 (3 x CHar.), 145.22 (Car.), 177.97 (CO-NH2), (EI) m/z (%): 329.49 ([M]+, 5.75), 106 (100). C20H31N3O: C, 72.91; H, 9.48; N, 12.75. Found: C, 72.85; H, 9.37; N, 12.83.
Biological Evaluation
The human tumor cell lines were obtained from NCI, MD, USA. All chemicals and solvents were purchased from Sigma-Aldrich.
Cell proliferation assay
The anticancer activities for compounds 5a-m and 6a-f were performed using a standard (MTT)-based colorimetric assay21.
Cell Cycle Analysis and Detection of Apoptosis in MCF-7 cell line
Studying the effect of compound 5i on the cell cycle progression using BD FASCCalibur after treatment with 3.25 μM of 5i for 48 h. Then, cell staining with an annexin V-FITC antibody and propidium iodide by FACS22.
Conclusion
Series of 1-(N-phenyl-2-(heteroalicyclic-1-yl)acetamido)cyclohexane-1-carboxamide derivatives (5a-m) and 1-(phenyl(heteroalicyclic-1-ylmethyl)amino)cyclohexane-1-carboxamide (6a-f) have been synthesized and screened or their anticancer activity. Compound 5i exhibited the most potent anticancer activity. It inhibited the MCF-7 cell line with IC50 value of 3.25 μM inducing apoptosis at PreG1 and arrested of the cell cycle at G2/M phase.
Conflict of interest
The authers having no conflict.
Acknowledgement
The authors thank the department of pharmaceutical chemistry, College of Pharmaceutical Science and Drug Manufacturing, Misr University for Science and Technology.
References
- Taher, A. T.; Helwa, A. A. Chem. Pharm. Bull. (Tokyo). 2012, 60, 521-530.
CrossRef - Szakács, G.; Paterson, J. K.; Ludwig, J. A.; Booth-Genthe, C.; Gottesman, M. M. Nat. Rev. Drug Discov. 2006, 5, 219-234.
CrossRef - O’Connor, R. Curr. Cancer Drug targets. 2009, 9, 273-280.
CrossRef - Abdelgawad, M. A.; Bakr, R. B.; Omar, H. A. Bioorg. Chem. 2017, 74, 82-90.
CrossRef - Newman-Tancredi, A.; Assie, M. B.; Martel, J. C.; Cosi, C.; Slot, L. B.; Palmier, C.; Rauly-Lestienne, I.; Colpaert, F.; Vacher, B.; Cussac, D. Brit. J. Pharmacol. 2007, 151, 237-252.
CrossRef - Moroni, A. Pneumonologie. 1985, 147, 62-74.
- Aboul-Enein, M. N.; El-Azzouny, A. A.; Attia, M. I.; Maklad, Y. A.; Aboutabl, M. E.; Ragab, F.; Abd El-Hamid, W. H. Int. J. Mol. Sci. 2014, 15, 16911-1935.
CrossRef - Abd-Allah, W. H.; Aboutabl, M. E.; Aboul-Enein, M. N.; El-Azzouny, A. A. Bioorg. Chem. 2017, 71, 135-145.
CrossRef - Aboutabl, M. E.; El-Hamid, W. H. A. Egypt. Pharm. J. 2015, 14, 196-203.
CrossRef - Wilson; Gisvold Text book of Organic and Medicinal and Pharmaceutical Chemistry.10th ed. 1998, 342.
- Liu, Y. Q.; Yang, H.; Tian, X. Chinese. J. Chem. 2006, 24, 785790.
- Teran, C.; Santana, L.; Uriarte, E.; Vina, D.; De Clercq, E. Nucleosides, nucleotides & nucleic acids. 2003, 22, 787-789.
CrossRef - Bohlin, L.; Rosen, B. Drug Discov. Today. 1996, 1, 343-351.
CrossRef - Damayanthi, Y.; Lown, J. W. Curr. Med. Chem. 1998, 5, 205-252.
- Cheng, W. H.; Shang, H.; Niu, C.; Zhang, Z. H.; Zhang, L. M.; Chen, H.; Zou, Z. M. Molecules. 2015, 20, 12266-12279.
CrossRef - Kushwaha, N.; Saini, R. K.; Kushwaha, S. K. Synthesis. 2011, 3, 203-209.
- Aboul‐Enein, M. N.; El‐Azzouny, A. M.; Ragab, F. A. F.; Hamissa, M. F. Arch. Pharm. 2017, 350, 1-12.
CrossRef - Suresh, N.; Nagesh, H. N.; Sekhar, K. V. G. C.; Kumar, A.; Shirazi, A. N.; Parang, K. Bioorg. Med. Chem. lett. 2013, 23, 6292-6295.
CrossRef - Tseng, C. H.; Tzeng, C. C.; Chiu, C. C.; Yang, C. L.; Lu, P. J.; Chou, C. K.; Liu, C. Y.; Chen, Y. L. Med. Chem. Comm. 2014, 5, 937-948.
CrossRef - Banday, A. H.; Kulkarni, V. V.; Hruby, V. J. Med. Chem. Comm 2015, 6, 94-104.
CrossRef - Mosmann, T. J. Immunol. Methods. 1983, 65, 55.
CrossRef - Manke, I. A.; Nguyen, A.; Lim, D.; Stewart, M. Q.; Elia, A. E.; Yaffe, M. B. Mol. Cell. 2005, 17, 37.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









