Solvent free Cationic Copolymerization of 2-Chloroethyl Vinyl Ether with Styrene Catalyzed by Maghnite-H+, a Green Catalyst
1Polymer Chemistry Laboratory, Department of Chemistry, Faculty of Exact and Applied Sciences, University of Oran1 Ahmed Ben Bella, BP El Menouar 1524, 31000 Oran, Algeria.
2University Dr Moulay Tahar, Bp138 Cite El Nasr Saida, Algerie.
Corresponding Author E-mail :malika.chabanipoly@laposte.net
DOI : http://dx.doi.org/10.13005/ojc/340229
Article Received on : January 10, 2018
Article Accepted on : February 22, 2018
Maghnite-H+ (Mag-H),proton-exchanged Montmorillonite, catalyzed the cationic copolymerization of 2-chloroethylvinylether (CEVE) with styrene (St) in a solvent free system. The objective is tosynthesize anend-group functionalized copolymer. The advantage of theMag-H lies in its capacity to solve ecological (green catalyst) and economic problems, and also to the fact that the reactions are carried out under moderate conditions, solvent freeand at room temperature.A kinetic study was carried out, and the products obtained were characterized using different methods of analysis, including IR, 1H and 13C NMR and viscosimetry.
KEYWORDS:Vinyl Ethers; Copolymers; Green Catalyst; Maghnite-H+; 2-Chloroethyl Vinyl Ether
Download this article as:| Copy the following to cite this article: Malika C, Rachid M, Mohammed B. Solvent free Cationic Copolymerization of 2-Chloroethyl Vinyl Ether with Styrene Catalyzed by Maghnite-H+, a Green Catalyst. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Malika C, Rachid M, Mohammed B. Solvent free Cationic Copolymerization of 2-Chloroethyl Vinyl Ether with Styrene Catalyzed by Maghnite-H+, a Green Catalyst. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44952 |
Introduction
Polyvinyl ethers are viscousgum to rubberysolids used for a wide variety of technological and industrial applications; they are particularly employed in manufacturing additives and nanometer membranes. They are used in telecommunications, in the synthesis of nonlinear optical (NLO) materials1, and also in electronics, in the production of light emitting diodes (LEDs)2.Chloroethyl Vinyl Ether (CEVE) is one of the most popular industrial products. CEVE is a special product because it possesses the functional group chloroethoxy that offers extremely varied possibilities for chemical modification and for anchoring functional groups. Hashimoto et al. employed CEVE as a starting material for preparing 2-vinyloxyethyl phthalimide by substituting the chlorine atom with potassium phthalimide.It was also used as a raw material in the synthesis of divinyl ethers 3, 4.
In recent years, both academic and industrial researchers have paid particular attention to green chemistry by developing organic and polymer syntheses that are respectful of the environment using renewable resources5. Solvent-free techniques, green solvents, water6, phase-transfer catalysts, or ionic liquids7 have replaced hazardous, toxic and volatile organic solvents 8. On the other hand, enzymes9-15, silica gel and silica-supported reagents 16, as well as clay minerals 17-25 are increasingly being used as catalysts. Furthermore, all reactions in this study are conducted at room temperature19-25.
Herein, we propose to use smectite clay, called Maghnite-H+20, as an efficient and recyclable green heterogeneous catalyst for the cationic copolymerization of ChloroethylVinyl Ether withStyrene at room temperature. The polymerization of vinyl ethers is widely known and has been used for a long time26; this chemical reaction can only occur by a cationic mechanism. This is the case of Mag-H which can initiate the polymerization reactions only cationically19-24. Moreover, Mag-H presents several advantages, among which are its high efficiency, easiness of handling, recyclability, high capacity to operate under mild conditions, and eco-friendliness.
Materials and Methods
Materials
2-Chloroethyl vinyl ether (CEVE) was commercially supplied (Sigma-Aldrich, 99%) and used as received. Styrene (St) (Sigma-Aldrich 99%) was washed with aqueous solution of NaOH (1N).Methylene chlorideanhydrous (Sigma-Aldrich, ≥99.8%) and methanol (SigmaAldrich, 99.8%)were used as received. Raw-Maghnite was procured from Bental (Algerian Society of Bentonite).
Methodology
Copolymerization of 2-Chloroethyl Vinyl Ether with Styrene using Maghnite-H+
Proton exchanged Montmorillonite clay called “Maghnite-H+” (Mag–H) is a non-toxic catalyst that was prepared according to the procedure reported in the literature19,20.
Bulk copolymerization of CEVE with Styrene was carried out in a heterogeneous system, at room temperature, using a percentage, by weight relative to monomers, of Mag-H as a catalyst (scheme 1). Copolymerization was carried out with different mole fractions (x = nCEVE/nST+ nCEVE), in several crimp tubes 7. The mixture prepared was stirred with a magnetic stirrer for a specific period of time. Then, a given quantity of methylene chloride was added and the mix obtained was dried under vacuum in order to eliminate Mag-H which precipitates in methanol. Finally, the copolymer was isolated and dried before analysis. The experimental conditions used are described in Table 1.
In a previous study on CEVE and St polymerization, with Mag-H (3% by weight) as catalyst, reaches maximum yield after 6 hours of reaction 22, 24.
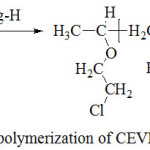 |
Scheme 1: Copolymerization of CEVE with Styrene Click here to View scheme |
Table 1: Experimentalconditions for the copolymerization of CEVE with Styrene (St) catalyzedby Mag–H (1%) at room temperature. Time: 6h.
|
Mole fraction |
nCEVE(mmol) |
nSt(mmol) |
Yield (%) |
|
0.2 |
4.70 |
18.78 |
31,88 |
|
0.3 |
4.70 |
10.95 |
36.29 |
|
0.4 |
9.39 |
14.09 |
54.78 |
|
0.5 |
9.39 |
9.39 |
57.45 |
|
0.6 |
9.39 |
6.24 |
63.15 |
|
0.7 |
18.78 |
8,07 |
69.38 |
|
0.8 |
18.78 |
4.70 |
71.50 |
Apparatus
In order to characterize our products, analytical techniques such as 1 H-NMR, 13 C-NMR and IR were used. Viscosimetry was employed for the kinetic reactions.
1H-NMR and 13C-NMR
The proton and carbon NMR spectra were recorded on a BRUKER AC 300MHZ apparatus. This technique allowed identifying the products obtained. The analysis was carried out in deuterated chloroform.
Infrared IR
ATR-FTIR absorption spectra were recorded on an Alpha Bruker ATR-FTIR spectrometer, in a range extending from 400 to 4000 cm-1.
Viscosimetry
The intrinsic viscosity was measured at 25 °C, in methylene chloride, on anUbbelohdecapillary viscosimeter, Viscologic TI.1, version 3-1.
Characterization
Three mole fractions, 0.2, 0.5 and 0.8 were analyzed by 1H NMR and one by13CNMRspectroscopy. Figures 1 and 2 show 1H NMR spectrumsand figure 3 shows 13C NMR spectrum.
Nuclear Magnetic Resonance (1H-NMR) analysis
 |
Figure 1: 1H NMR spectrum of the CEVE-Styrene copolymer in DCCl3 (300MHz). (x = 0.8)
|
 |
Figure 2:1H NMR spectrum of the CEVE-Styrene copolymer in DCCl3 (300MHz). |
Results and discussion
The 1H NMR analysis indicates the presence of the main protons corresponding to the (CEVE-Styrene) copolymer.The 1H NMR spectrum of the copolymeris almost the same to that reported in the literature by Cramail27.Peaks (i,h), observed between 5.2 and 5.80 ppm, correspondtoH-vinyl of styrene of the chain end.
The number of CEVE and St units per chain in copolymer and were determined by 1H NMR (figure 2) from the ratio, on the one hand, between the -CH2-CH2-Cl methylene protons, the CH-O methine proton and aromatic proton of styrene and, on the other hand, between the aromatic proton of styrene and H-vinyl of styrene of the chain end. Results are shown in table 2.
Table 2: Number of CEVE and St unitsand values
|
X |
m/n |
N |
m |
|
|
0.2 |
2 |
24 |
48 |
7611,12 |
|
0.5 |
6 |
9 |
54 |
6687,81 |
|
0.8 |
17 |
1 |
17 |
1914,48 |
Results show that in this copolymerization, CEVE is much more reactive than styrene. Values of rl = 36, r2 = 3, and 66 ≥ r1≥ 9, 0.125 ≥ r2 ≥ 0.01were reported respectively by Kanoh et al.28and Brown and Pepper 29for this system.
Nuclear Magnetic Resonance (13C-NMR) analysis
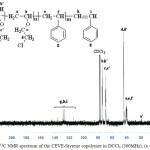 |
Figure 3: 13C NMR spectrum of the CEVE-Styrene copolymer in DCCl3 (300MHz). (x = 0.5).
|
Figure 3 shows 13C NMR spectrum of the copolymer.The 13C NMR spectrum shows the chemical shifts associated with the CEVE-St copolymer. Carbons of the CEVE unit resonate between 35 and 80 ppm. As forcarbons of the aromatic ring, they resonate between 110 and 140 ppm.13C NMR Spectrum shows the difference between the reactivities of the two monomersby comparing the intensities of g and d peaks.
ATR-FTIR Spectra
Figure 4 shows the ATR-FTIR spectrum of the copolymer (CEVE-Styrene)
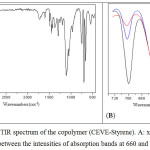 |
Figure 4: ATR-FTIR spectrum of the copolymer (CEVE-Styrene). A: x = 0.2 and B: comparison between the intensities of absorption bands at 660 and 700 cm-1. |
Figure 4 shows the ATR-FTIR spectrum of the copolymer.The FTIR spectra indicate the presence of St and the CEVE units.As for the Strepeatingunit, aromatic C–H stretching occurs between 3000 and 3105 cm-1,aromatic C=C stretching occurs at about 1600 cm-1andStabsorption of the out-of-plane ringdeformation band occurs at about 700 cm-1.Concerning the CEVErepeating unit, spectrum illustrates bands corresponding to C-Cl bond at about 660 cm-1 and C–O–C stretching of vinyl ether between 1090 and 1110 cm-1. FTIR results from CEVE with St copolymerization showed that characteristic absorbance bands at about 700, the strongest IR band of St unit, and at 660 cm-1, IRband of CEVE unit, could be used to follow the evolution of each unitin copolymer.Indeed, the comparison, between the intensities of absorption bands at 660 and 700 cm-1, shows the great difference between the reactivities of St and CEVE (figure 4, B andtable 3).
Table 3: Comparison between the intensities of absorption bands at 660 cm-1 and 700 cm-1.
|
X |
IC-H (Aromatic) |
IC-Cl |
IC-Cl/IC-H |
|
0.2 |
0.5615 |
0.3990 |
0.711 |
|
0.5 |
0.2450 |
0.4550 |
1.86 |
|
0.8 |
0.0867 |
0.4144 |
4.78 |
I: intensity of absorption bands
Viscosimetricanalysis
In order to understand the physical nature of the copolymers obtained, the evolution of their intrinsic viscosities was followed, in the solution of dichloromethane, at a temperature of25 °C. The results obtained are grouped in figure 6.
Kinetic study
The aim of the present study is to vary the amount of catalyst in order to examine its influence on the course of the living copolymerization.
Effect of the amount of Maghnite-H+ on the yield
This study aims to know the behavior of our copolymer system, namely (2-Chloroethyl Vinyl Ether – Styrene) + Maghnite-H+
Description of experiment
In order to determine the effect of the amount of the catalyst on the yield, three (03) series of experiments were conducted, by varying the percentages of the amount of Mag-H, as 1%, 2% and 3%.The results obtained are presented in figure5.
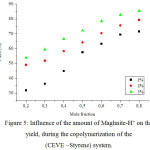 |
Figure 5: Influence of the amount of Maghnite-H+ on the yield, during the copolymerization of the(CEVE –Styrene) system.
|
Figure 5 shows the effect of the amount of Mag-H on the copolymerization rate of CEVE with St. Indeed, using various amounts of Mag-H, 1, 2, and 3 % by weight, the copolymerization was carried in bulk at room temperature. The copolymerization rate increased with the amount of Mag-H, in which the effect of Mag-H as a cationic catalyst of the copolymerization is clearlyshown. Similar results are obtained by Beloufa et al.30 And Bouchama et al.31in, respectively,the copolymerization of 1,3,5-trioxane with 1,3-dioxolaneand e-caprolactone with tetrahydrofuran catalyzed by Mag-H.This increase in yield is due to an increase in the number of ‘‘initiating active sites’’ responsible of inducing polymerization, a number that is proportional to the amount of catalyst.
Effect of the amount of Maghnite-H+ on intrinsic viscosity
The different samples obtained from the different experiments were subjected to the viscosimetric analysis.The results are shown in figure 6. As is shown, on the one hand,the increase of the catalyst amount led to the decrease of intrinsic viscosity. This is comparable to thatof the most active catalyst systems. The explanation for the decreased of intrinsic viscosity is that the chain transfer reaction rate is faster than that of the propagation reaction.On the other hand, the decrease in the quantity led to the increase of the intrinsic viscosity, this is due to the high reactivity of CEVEwhich leads to the formation of oligomers 22.
![Figure 6: Influence of the amount of Maghnite-H+ on the viscosity [η] during the copolymerization of the system (CEVE -Styrene)](http://www.orientjchem.org/wp-content/uploads/2018/04/Vol34No2_Sol_Cha_fig6-150x150.jpg) |
Figure 6: Influence of the amount of Maghnite-H+ on the viscosity [η] during the copolymerization of the system (CEVE -Styrene)
|
From the results obtained, it can be said that the intrinsic viscosity decreases as the amount of Maghnite-H+ goes up.Moreover, the intrinsic viscosity increases with increasing percentage (%) of the most reactive comonomer.In this case, styrene (r1 = 160) is more reactive than CEVE (r2 = 0.07), and this difference in reactivity between the two monomers is due to the fact that the two types of active centers react with the most reactive monomer. The reaction mixture is therefore enriched with styrene, which is more reactive than CEVE. This causes the yield to decrease, while the intrinsic viscosity increases.
Proposed Mechanism
The copolymerization is proposed undertaking in a way as shown in Scheme 2. Mag-H performs as an environmentally friendly solid catalyst. It was regarded as an initiator an activator 19-24,31.The copolymerization of CEVE with St is initiated,firstly, by proton addition from Mag-H to CEVE (Eq.1), due to the most reactivity of CEVE compared to the St, and the Maghnitetakes place as counter-ion. Propagation thentakes place by conventional cationic mechanism (Eq.2). Secondly, reaction between growing macrocationand St occurs (Eq.3).The copolymerization ends by proton transfer to St and/or toMaghnite produced by unsaturationas shown in the termination reaction(Eq.4).
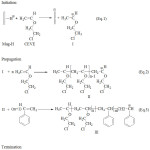 |
Scheme 1a |
We suppose that there was formation of adouble bond at the end of the chain of the copolymer byspontaneous transfer.
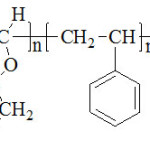 |
Scheme 2 Click here to View scheme |
Conclusion
The main goal was the synthesis of copolymers using an environmentally friendly solid catalyst. Our study aimed particularly at investigating the copolymerization of vinyl monomers, such as 2-chloroethylvinylether (CEVE) and Styrene (St). The cationic bulk polymerization, catalyzed by Mag-H,occurstelechelic copolymer which is characterized by various methods, like 1H-NMR, 13C-NMR, FTIR, and Viscosimetry.1H-NMR, 13C-NMR confirmed the production of poly (CEVE-co-Styrene) with styrenic unsaturation end chain which gives a monotelechelic copolymer.
The kinetic study of cationic copolymerization in the presence of Mag-H makes it possible to draw the following conclusions:
Bulk copolymerization is possible,
Yield increases with increasing amount of Mag-H,
Yield decreases with increasing percentage of the most reactive comonomer,
Increasing the amount of Mag-Hleads to a decrease in the intrinsic viscosity,
Intrinsic viscosity increases while increasing the percentage of the most reactive comonomer.
Acknowledgements
This work was supported by the Algerian Ministry of University (MESRS) and by the General Direction for Scientific Research and Technological Development (DGRSDT).
References
- Lee,J.Y.;Kim, M.J.;Synthesis of Highly Crosslinked Poly(vinyl ethers) Containing the Nonlinear Optical Chromophore Oxybenzylidenemalononitrile and Oxycyanocinnamate,Bull.Korean.Chem. Soc.,1999,20(2), 253-256.
CrossRef - Heischkel, Y.; Schmidt,H.W.; Synthesis of ABC-triblock copolymers for light emitting diodes.Macromol.Chem. Phys.,1998,199, 869-880.
CrossRef - Hashimoto,T.; Ibuki, H.; Sawamoto, M. and Higashimura,T. Living cationic polymerization of 2-vinyloxyethyl phthalimide synthesis of poly(vinyl ether), J. Polym. Sci. Polym. Chem.,1988, 3374.
- Hashimoto,T.;Watanabe, K.;Kodaira, T. Cationic Cyclopolymerizationof 1,2-bis(2-vinyloxyethoxy)benzenes :Introduction of Bulk Substituents to Increase Cyclopolymerization Tendency, J.Polym. Sci. Part A:Polym.Chem.,2004,42(13),3373–3379.
CrossRef - J.Clark andD.M.A. Macquarrie, Handbook of Green Chemistry,Oxford: Blackwell.,2002,1-531.
- Li, C.J. Organic Reactions in Aqueous Media with a Focus on Carbon-Carbon Bond Formations: ADecade Update,Chem. Rev.,2005,105, 3095-3165.
CrossRef - Koichi,M. Green Reaction Media for Organic Synthesis,Weiheim: Wiley-VCH.,2005, 9-58.
- Tanaka, K.Solvent-Free Organic Synthesis,Weinheim: Wiley-VCH.,2003, 1-402.
CrossRef - Chaudry, A.K.;Beckman, E.J.;Russell,A.J. Rational control of polymer molecular weight and dispersity during enzyme-catalyzed polyester synthesis in supercritical fluids,J. Am. Chem. Soc.,1995,117(13), 3728–3733.
CrossRef - Svirkin, Y.Y. ;Xu,J.; Gross, R.A.; Kaplan, D. ; Swift,G. Enzyme-catalyzed stereoelective ring-opening polymerization of a-methyl-b-propiolactone,Macromolecules., 1996,29(13),4591–4597.
CrossRef - Banerjee,S.;Ramannair, P.; Wu, K.;John, V.T.;McPherson, G.;Akkara, J. ;Kaplan, D. In Enzymes in Polymer Synthesis; R.A. Gross, D.L.Kaplan, G.S.Swift, Eds.; ACS Symposium Series 684; Am. Chem. Soc.: Washington, DC., 1998, 125.
- Chen,H.N.;Gu,Q.M. Biotransformation of polysaccharides. In: Wang, P.G. and Bertozzi,C.R.Edit.,Glycochemistry: principles, synthesis and applications. (New York: Marcel Dekker.,2001, 567.
- Gubitz,G.M.;CavacoPaulo,A. New substrates for reliable enzymes: enzymatic modification of polymers. Curr.Opin.Biotech.,2003,14(6), 577–82.
- Javie,A.W.P.;Overton,N.;St Pourcain,C.B. Enzyme catalysed modification of synthetic polymers,J. Chem. Soc. Perkin Trans.,1999,1, 2171–2176.
- Puskas,J.E.;Sen, M.Y.;Seo,K.S. Green polymer chemistry using nature’s catalysts: enzymes. J.Polym. Sci.Part A: Polym. Chem.,2009,47, 2959–76.
CrossRef - Mahdavinia,G. H.;Rostamizadeh,S.;Amani, A.M.; Mirzazadeh, M. NH4H2PO4/SiO2: a recyclable, efficient heterogeneous catalyst for crossed aldol condensation reaction, Green Chem. Lett. Rev.,2012,5(3), 255-281.
CrossRef - Vaccarir, A. Clays and catalysis: a promising future, Appl. Clay Sci., 1999,141, 61–198.
- Nagendrappa, G. Organic synthesis using clay and clay-supported catalysts, Appl. Clay Sci.,2011,53, 106–138.
CrossRef - Harrane,A.;Meghabar,R.; Belbachir, M.A Protons Exchanged Montmorillonite Clay as an Efficient Catalyst for the Reaction of Isobutylene Polymerization, Int. J. Mol. Sci.,2002,3, 790-800.
CrossRef - Meghabar,R.; Megherbi, A.; Belbachir, M. Maghnite-H+, an ecocatalyst for cationic polymerization of N-vinyl-2-pyrrolidone, Polymer., 2003, 44, 2397
CrossRef - Megherbi,R.; Belbachir, M.;Meghabar, R. Maghnite-H+ as a Cationic Catalyst in the Synthesis of Poly(1,3-dioxolane) and a,w-Methacryloyloxy-Poly(1,3-dioxolane), J. Appl.Polym. Sci.,2006,101, 78–82.
CrossRef - Chabani,M.; Yahiaoui, A.;Hachemaoui, A.; Belbachir,M. New approach for the polymerization of 2-chloroethyl vinyl ether using Maghnite clayas eco-catalyst, J App. Polym. Sci., 2011,122(3), 1800-1806.
- Benkenfoud, K.; Harrane, A.; Belbachir, M. Ring opening polymerization of tetrahydrofuran catalysed by Maghnite-H+, Chin. J. Polym.Sci.,2012,30(1), 56-62.
CrossRef - Baghdadli,M.C.; Meghabar, R. and Belbachir, M.Acid-Activated Algerian Montmorillonite as Heterogeneous Catalysts for Cationic Polymerization of Styrene,Asian J. Chem.,2016,28(6), 1197-124.
CrossRef - Ouis, N.;Benharrats, N.;Belbachir, M. Synthèses de polytétrahydrofurane catalysées par le kaolin de Tamazert, C. R. Chimie.,2004,7, 955-962.
CrossRef - Wislicenus,J. Ueber Vinyl Ethyl Ether, Ann. Chem.,1978,192, 106.
- Cramail,H.;Schappacher, M.;Deffieux, V. The α-Halogeno Ether Function: Formation and use in the Design of Tailor –made polymers, Makromol. Chem.,1992,193, 2793,
CrossRef - Brown,G. R. and Pepper, D. C.Cationic Copolymerisation of 2-Chloroethyl Vinyl Ether with Styrene and with a-Methyl styrene, J. Chem. Soc.,1963,0, 5930-5933 .
CrossRef - Kanoh,N.;Gotoh,A.;Higashimura, T. andOkamura,S. Rate constant of propagation reaction in stationary state of cationic polymerization. Part V1. Copolymerization, Makromol.Chem.,1963,63, 106.
CrossRef - Beloufa,K.;Sahli, N.;Belbachir, M. Synthesis of copolymer from 1,3,5-trioxane and 1,3-dioxolane catalyzed by Maghnite-H+, J Appl. Polym. Sci., 2010,115(5), 2820-2827.
CrossRef - Bouchama,A.; Ferrahi, M.I.;Belbachir, M. Synthesis of Poly (e-Caprolactone-Co-Tetrahydrofuran) by an Acid Exchanged Montmorillonite Clay, Macromol. Symp.,2011,308, 122–125.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










