Seasonal Influences on the Levels of Particulate Cd, Cr and PB in Kuantan River, Pahang
Kamaruzzaman Yunus1 , Fikriah Faudzi1, Mohd. Fuad Miskon1 and Mohd Mokhlesur Rahman2
, Fikriah Faudzi1, Mohd. Fuad Miskon1 and Mohd Mokhlesur Rahman2
1Kulliyyah of Science, International Islamic University Malaysia, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota, 25200 Kuantan, Pahang, Malaysia.
2Institute of Community Health development and Quality of Life ( i-CODE), University Sultan Zainal Abidin, Kuala Terengganu, Terengganu.
Corresponding Author E-mail: mokrahman@unisza.edu.my
DOI : http://dx.doi.org/10.13005/ojc/340226
Article Received on : February 03, 2017
Article Accepted on : December 31, 2017
Temporal and spatial variations of selected toxic elements distributions were studied in the Kuantan River waters. Water samples from 9 sampling stations were taken from downstream of the estuary towards the upstream of Kuantan River during the rainy and dry season. Particulate Cd, Cr and Pb were filtered, dried, weighed and analyzed using Teflon Bomb digestion processes. The concentration of particulate Pb, Cd and Cr were in the range of 0.646 to 174.859 mg L-1, 6.047 to 271.497 mg L-1 andND to 14.480 mg L-1, respectively. Particulate metals obtained mostly higher in May 2012 (dry season) while Cd and Cr were found above the limits authorize by International Commission for the Protection of the Rhine against Pollution (ICPR). Correlation matrix between different parameters shown that most of the parameters were significantly correlated with each other.
KEYWORDS:Suspended Particulate Matter; Toxic Metals; River Water; Cadmium; Chromium; Lead
Download this article as:| Copy the following to cite this article: Yunus K, Faudzi F, Miskon M. F, Rahman M. M. Seasonal Influences on the Levels of Particulate Cd, Cr and PB in Kuantan River, Pahang. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Yunus K, Faudzi F, Miskon M. F, Rahman M. M. Seasonal Influences on the Levels of Particulate Cd, Cr and PB in Kuantan River, Pahang. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=43826 |
Introduction
Riverine environments often have considerably higher concentrations of trace components such as nutrients and many transition metals and metalloids compared to marine environments. At present, there are increasing number of river water that neither can be used nor treated and were classified as highly polluted. DOE (2010) report shows that amongst the 143 river basins that being examined, 64% (91) are categorized as clean, 31% (45) are slightly polluted and 5% (7) are polluted. Environmental Quality Act (1974) of pollution according to Interim National Water Quality Standards (INWQS) was established in order to monitor the water quality management in Malaysia. All pollutants discharge from industries is controlled by EQA Regulation under INWQS and monitored by authority. Metals ions known as to be toxic as their tissue may degraded which are dangerous and detrimental to nature compared to other various inorganic and organic water pollutants. Lead, chromium and cadmium contamination are quite well known (5). Therefore, toxic metals demand close monitoring since they are relatively unstable, carcinogenic and bioaccumulative (19).
Moreover, toxic elements may dissolved in wastewaters and discharged into surface waters and later will be concerted as they enter the food chain. Metals that leach into ground waters will contaminate drinking water wells and harm the adjacent community. Information on the level of toxic elements in Malaysia aquatic environment is inadequate, which constituted of a few studies. Development along the east coast of Malaysia rapidly increasing with factories and industrial activities apart from oil and gas-related industries that knowingly founded in the states of Pahang and Terengganu. Kuantan has a humid tropical climate with seasonal monsoon rain where two monsoon periods prevail i.e Northeast Monsoon (October to March) and Southwest Monsoon (May to September). Hence, the aim of this study was to determine the spatial and temporal variations of particulate elements during the monsoon (wet) and non-monsoon (dry) season.
Materials and Methods
Kuantan River Basin covers an area of 1,586 km2 and is approximately 80 km long (DOE 2010). Kuantan River flows through two mukims i.e. Ulu Kuantan and Kuala Kuantan and flowing out to the South China Sea. Major settlements in the basin include Kuantan town, Lembing River and Gambang. All apparatus including glasswares and plastic wares that were used in the laboratory and during the sampling were immersed in 10% nitric acid (HNO3) for at least 72 hours, rinsed with deionized water and were dried in the oven for 24 hours. Nylon membrane filters paper (47 mm diameter, Whatmann) with pore size of 0.45 µm were immersed in 10 % HNO3, washed with deionized water and were dried in class 100 laminar flow hood (2). The Nylon membrane filters were weighed for several times until a constant weight obtained.
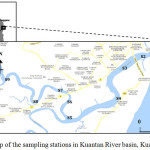 |
Figure 1: Map of the sampling stations in Kuantan River basin, Kuantan, Pahang |
Surface and bottom water samples were collected in May 2012 and October 2012 from 9 stations using Van-Dorn Water Sampler. Hydrological general physicochemical parameters such as salinity, dissolved oxygen (DO), pH and temperature were measured in-situ using YSI Hydrolab multisensory probe. Water samples were stored in acid-washed polyethylene bottles (1L) and were kept in ice during the transportation to the laboratory. At the laboratory, within 24 hours, water samples were filtered past Nylon millipore filters (0.45 µm pore size) that were pre-weighed by using a low-pressure vacuum pump. The membrane filter that contains wet suspended particulate samples were dried under laminar flow for several days, weight and re-weighed until constant weight obtained and labeled as the particulate sample.
Samples were transferred into Teflon bomb for digestion (8, 14). Samples were added together with 1.5 mL of mixed acid with the ratio of 3.5 HNO3: 3.5 Hydrochloric acid (HCl): 3 Hydrofluoric acid (HF) in a Teflon vessel and heated for 5 to 7 hours at 150°C. After cooling session, digested sample were added with 3 mL of mixed of boric acid (H3BO3) and EDTA and were heated again for 5 to 7 hours at 150°C in the microwave oven. Then sample were cooled down before transferred into 10 mL test tube and added for dilution up to 10mL with Milli-Q water. The same digestion procedures were done for filters without particles and labelled as procedural blanks. Samples digestion was performed in duplicate to certify the reliability of the method. Blanks and quality control samples that were prepared from standard solutions were analyzed for every ten samples so as to check the sample accuracy. Recovery test for particulate metals was conducted using SRM 1646a Estuarine Sediment. The percentage of recovery obtained were ranged 98.230% for Cd, 100.830% for Cr and 104.920% for Pb. Concentration of particulate metal concentration was calculated using the formula:
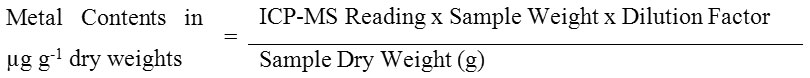
Remarks:
Dilution factor: 10 times
Results and Discussion
Salinity in Kuantan River decreased from estuary to the upper streams stations. Higher salinity was recorded in May 2012 than October 2012 with the mean value of 21.450 ± 9.900 ‰ and 20.080 ± 10.600 ‰, respectively. Mean temperature was found higher in May 2012 with value of 29.790 ± 0.630 °C while heavy rainfall was recorded in October 2012. This indicates that May 2012 was the dry season and October 2012 was the wet season. pH also showed higher mean value in May 2012 than October 2012 with 7.110 ± 0.580 and 6.550 ± 0.500, respectively. Meanwhile, dissolved oxygen concentrations varied between 4.460 and 7.670 mg L-1 that is indicative of adequate oxygenated river waters. Mean values for physicochemical parameters are listed in Table 1.
Cadmium concentrations ranged from below 0.478 to 11.594 mg L-1 as shown in Table 1. Maximum values were encountered at the upstream station of Kuantan River. Mean concentration of particulate Cd was found relatively higher with value of 4.058 ± 2.432 mg L-1 in May 2012 compared to October 2012 (2.184 ± 1.678 mg L-1). Concentrations of particulate Cd throughout the sampling periods exhibited minor fluctuation with mostly increased concentration from the downstream estuary to the upstream station along the flow path (Figure 2). Sudden increased was observed at the surface layer of Station 7 and 8 in May which located at the city center, though the concentration observed was slightly dropped at Station 9. Concentrations at the surface layers were higher than the bottom layers. Particulate Cd in Kuantan River waters were found above the limit of 1 mg L-1 that approved by International Commission for the Protection of the Rhine againts Pollution (6).
Table 1: Summary statistic of element concentration, water quality parameters and rainfall data of Kuantan River basin during sampling period
| Month | Element | Average | SD | Min | Max | |
| May-12 | Cd (mg/L) | 4.058 | 2.432 | 0.849 | 11.594 | |
| Cr (mg/L) | 92.183 | 30.84 | 36.895 | 168.19 | ||
| Pb (mg/L) | 76.642 | 36.542 | 21.057 | 147.43 | ||
| pH | 7.11 | 0.58 | 6.66 | 7.82 | ||
| Salinity (ppt) | 21.45 | 9.9 | 0.33 | 34.3 | ||
| DO (mg/L) | 5.4 | 0.64 | 4.46 | 6.61 | ||
| Temperature (°C) | 29.79 | 0.63 | 28.15 | 30.78 | ||
| Rainfall mm (inches) | 169 (7.24) | – | – | – | ||
| Oct-12 | Cd (mg/L) | 2.184 | 1.678 | 0.478 | 8.182 | |
| Cr (mg/L) | 58.867 | 25.349 | 6.047 | 111.576 | ||
| Pb (mg/L) | 38.325 | 18.883 | 7.77 | 82.32 | ||
| pH | 6.55 | 0.5 | 6.02 | 7.43 | ||
| Salinity (ppt) | 20.08 | 10.6 | 0.09 | 33.57 | ||
| DO (mg/L) | 6.25 | 0.8 | 4.71 | 7.67 | ||
| Temperature (°C) | 28.92 | 1.01 | 27.242 | 30.05 | ||
| Rainfall mm (inches) | 283.9 (10.996) | – | – | – |
Particulate chromium showed distinct distributional characteristics and ranged widely, from 6.047 to 168.190 mg L-1 as shown in Table 1. Throughout the sampling periods, particulate Cr recorded the highest concentration compared to Cd and Pb. The mean concentration of particulate Cr found in May 2012 was higher than October 2012 with a value of 92.183 ± 30.840 mg L-1 and 58.867 ± 25.349 mg L-1, respectively (Figure 3). The average Cr concentration in Kuantan River water was measured to be higher than the ICPR limit of 100 mg L-1 of Cr (6). Likewise Cd and Cr, maximum values of particulate Pb encountered at the upstream stations, and occasionally in the vicinity of the Kuantan city center. Multifold increasing was observed with distance from river mouth to the upstream along the flow path. The highest mean concentration of particulate Pb with mean value of 76.642 ± 36.542 mg L-1 was relatively found in May 2012, while mean concentration in October 2012 was 38.325 ± 18.883 mg L-1. Throughout the sampling periods, particulate Pb exhibited higher concentrations on the surface layers than bottom layers as shown in Figure 4. Mean concentrations of particulate Pb are lower than ICPR limit of 100 mg L-1 (6) and listed in Table 1.
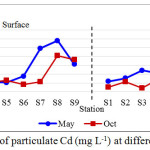 |
Figure 2: Concentrations of particulate Cd (mg L-1) at different sampling periods |
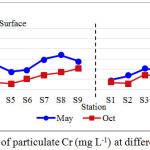 |
Figure 3: Concentrations of particulate Cr (mg L-1) at different sampling periods |
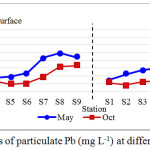 |
Figure 4: Concentrations of particulate Pb (mg L-1) at different sampling periods |
A positive and significant correlation was found in pH and Cr while less significant was found between temperature and Cr (p > 0.05) as shown in Table 2. Inversely, salinity was negative and significantly correlated with Cd and Pb though less significant with Cr. DO observed was less significant and negatively correlated with Cr and Pb. Pearson correlation matrixes of suspended particulate metals are given in Table 3. Overall, correlation analysis showed that most of the particulate elements had a strong and positive relationship with each other. A good positive correlation that was found between almost all heavy metals shows that there were a common source or at least one major source for all of them and may reflect similar behavior. Zhou et al. (2003) whom also found significant inter-element relationships in SPM claimed that some trace metals have similar reactivity towards biological and non-biological particle and, therefore, possible for some of them will have their particulate–bound concentrations closely related to each other.
Table 2: Correlation analyses of physicochemical parameters with particulate elements
| Salinity | Temperature | pH | DO | |
| Cd | -.240** | -0.101 | -0.02 | 0.073 |
| Cr | -.162* | .147* | .201** | -.157* |
| Pb | -.316** | 0.002 | -0.01 | -.171* |
**Correlation is significant at the 0.01 level (2-tailed).
*Correlation is significant at the 0.05 level (2-tailed).
Table 3: Pearson’s correlation matrix indicating the overall elements association within particulate metals
| Cd | Cr | Pb | |
| Cd | – | .366** | .677** |
| Cr | – | – | .656** |
**Correlation is significant at the 0.01 level (2-tailed).
Suspended particulate matter of Cd, Cr and Pb showed multifold increasing trends from the downstream estuary to the upstream stations along the river flow path. Increasing concentration in particulates metals after the monsoon season subsides was due to adsorption onto re-suspended or elevation of natural particles that formerly present. Study by Kern and Westrich (1995) of the Neckar River in Germany, showed higher elements that originated from anthropogenic found in fine sediment (~20 mm) compared to the coarse particle. Elements such as Fe and Mn has higher concentration in the coarse particles since their main sources consist of soil. Therefore, from the trend observed, two particulate matter sources were discovered i.e flocculation processes derived particles from urban and industrial wastes or natural particles instigated by erosion.
Fine particles originated from the run-off of the sewage discharges may be the main source of this metal. During the low current of the river, there are high probabilities the particles were adsorbed. Heavy rainfall during monsoon may result to adsorption of large soluble fraction of Cd onto suspended solids throughout the dry season. Ouseph (1992) also discovered higher concentration in particulate metal content during the low rainy season. Desorption of Cd with an increase in salinity due to the formation of stable chloro-complexes have been reported by Che et al. (2003). Desorption of Cd from particles in the estuarine site may be the cause for the metal-enrichment in May 2012. During the wet season, enormous large particles as well as metal-deficient smaller particles were washed off by the monsoon shower to the river waters. Then, the metals were mixed with metal-rich particles causing a low concentration in the particulate Cd. Study by Piatina and Hering (2000) found that Cd was connected with finer particles for example clay minerals.
The main sources of elevated concentration of particulate Cr possibly came from the riverine input and run-off from terrestrial. Flocculation are higher since Cr has a great microparticulate element while the river water enters the estuary. Abu-Saba and Flegal (1995) study in San Francisco Bay claimed that industrial and municipal discharges and weathering contributed to the enrichments of particulate Cr in suspended matter. Moreover, the study by Jarvie (2000) shown that Cr has greater particulate and microparticulate elements, low solubility whereby during the particulate splitting process, colloidal combination may have followed. Although no clear spatial pattern could be established, maximum values of particulate Cr were always found at the stations situated nearby the city centers (S3, S4 and S5) as well as upstream stations (S8 and S9) corresponding to low salinity values. The potential source of Cr in the Kuantan River is possibly from the boats paints that consist higher amounts of Cr in the form of zinc chromate instigating the leaching of substantial quantities of the metal to the water. The nearness of the stations to the city centers, residential areas and the domestic waste discharges into the Kuantan River might have increased the metal content.
Meanwhile, during the heavy rainfall, particulate Pb that entered to the river systems in the soluble form were precipitated onto particles subsequent after the monsoon period ended and resulted in high concentration in May 2012. Higher particulate component of Pb were probably because of the low propensity to create allied with particles by scavenging (7). Furthermore, experimental study carried out by Shulkin and Bogdanova, (1998) and field studies by Westerlund et al. (1986) confirmed that Pb has high affinity to the solid phase. Another mechanism, dissolved Pb that released from sediments were re-adsorbed onto the suspended particles also may affects the particulate Pb concentration. Other aspects that may involves in higher concentration of particulate Pb includes shipping wastes, discharges from petroleum industries, land run-off sources, and natural sources such as biological activities and effects of wind. In the Kuantan River, one of the major source of Pb were the emanations from recurrent boating activities. Further monitoring need to be done in Kuantan River as the rapid development from adjacent city centers may increase the pollution of river water.
Conclusion
The concentration of the particulate elements was found higher in May and also showed increasing trend from downstream estuary to the upstream stations. Agricultural, boating and recreational activities, effluents and domestic wastes from surface run-off and drains may contributed to the significant amounts of particulate metals in the Kuantan River. Further monitoring need to be done in the river as the rapid development from adjacent city centers may increase the pollution of river water.
Acknowledgement
The author thanks the International Islamic University Malaysia for providing Research Initiative Grant Scheme (RIGS) ID 16-321-0485 and also appreciation to the lab assistant of International Islamic University Malaysia for their treasured assistance and hospitality.
References
- Abu-Saba, K.E.; Flegal, A.R. Cr in San Francisco Bay: superposition of Geochemical processes cause complex spatial distribution of redox species, J. Marine Chem. 1995, 49, 189-199.
CrossRef - APHA- Awwa- Wef. In: Clesceri, L.S., Greenberg, A.E., Eaton A.D. (Eds.), Standard Methods for the Examination of Water and Wastewater. 20th Edition, American Public Health Association, Washington. 1998, 1200.
- Che, Y.; He, Q.; Liu, W.Q. The distribution of particulate trace metal and its indication to the transfer of sediments in the Changjiang estuary and Hangzhou Bay, China. Marine. Pollution Bulletin. 2003, 46, 123-131.
CrossRef - Department of Environment. River Water Quality Status, Official Website of Department of Environmental. 2010, http/www.doe.gov.my
- Forstner, U.; Wittmann, G.T.W. Metal Pollution in the Aquatic Environment. Springer Verlag, 1983, 18.
- ICPR. Methodology to implement item A.2 of the Rhine Action Programme related to water quality objectives, prepared at Lenzbourg on 2 July 1991. International Commission for the Protection of the Rhine against Pollution, Koblenz, Germany. 1991, PLEN 3/91.
- Jarvie, H.P.; Neal, C.; Burton, L.O.; Tappin, A.O. Patterns in trace element chemistry in the freshwater tidal reaches of the River Trent. Sd. Total Environment, 2000, 317-333.
- Kamaruzzaman, B.Y. Geochemistry of the marine sediments: Its Paleoceonographic Significance. Unpublished Doctoral Dissertation. 1999, Hokkaido University, Japan.
- Kern, U.; Westrich, B. Sediment contamination by heavy metals in a lock-regulated section of the River Neckar. Marine Freshwater Research. 1995, 46:101–106.
- Ouseph, P.P. Dissolved and particulate trace metals in the Cochin estuary. Marine Pollution Bulletin. 1992, 24, 186–192.
CrossRef - Paucot, H.; Wollast, R. Transport and transformation of trace metals in the Scheldt estuary. Mar. Chem. 1997, 58, 229–244.
CrossRef - Piatina, T.B.; Hering, I.G. Direct quanfification of metal-organic inaction by size-exclusion chromatography (SEC) and inductively coupled plasma mass spectrometry (lCP-MS). J. Envt Quality. 2000, 29, 1839-1845.
CrossRef - Shulkin, V.M.; Bogdanova, N.N. Mobilization of metals from riverine suspended matter in seawater. Marine Chemistry. 2003, 83, 157-167.
CrossRef - Tsunogai, S.; Yamada, M. 226 Rain Bering Sea sediment and its application as a geochronometer. Geochemical Journal. 1979, 13, 231-238.
CrossRef - United Nations Environment Programme (UNEP). Final review of scientific information on lead. 2010. Chemicals Branch, DTIE.
- Westerlund, S.F.G.; Anderson, L.G.; Hall, P.O.; Werfeldt, A.; Van der Locff, M.; Sundby, B. Benthic fluxes of cadmium, copper, nickel zinc and lead in the coastal environment. Geochemistry. Acta. 1986, 50, 1289-1296.
- World Health Organization (WHO). Environmental Health Criteria for Cadmium. 1974.
- Zhou, J.L.; Liu, Y.P.; Abrahams, P.W. Trace metal behaviour in the Conwy estuary, North Wales. Chemosphere. 2003, 51, 429–440.
CrossRef - Zuane, J.D. Hand Book of Drinking Water Quality. Van Nostrand Reinhold, New York. 1990, 47-151.

This work is licensed under a Creative Commons Attribution 4.0 International License.









