Polyimide Composite Materials Containing Modified Nanostructured Boron Carbide
Elena Aleksandrovna Averina, Anton Sergeyevich Yegorov , Marina Vladimirovna Bogdanovskaya, Alyona Igorevna Wozniak and Olga Anatolevna Zhdanovich
, Marina Vladimirovna Bogdanovskaya, Alyona Igorevna Wozniak and Olga Anatolevna Zhdanovich
The Federal State Unitary Enterprise «Institute of Chemical Reagents and High Purity Chemical Substances of National Research Centre «Kurchatov Institute» 107076, Bogorodsky val, 3. Moscow. Russia.
Corresponding Author E-mail: egorov@irea.org.ru
DOI : http://dx.doi.org/10.13005/ojc/340217
Article Received on : December 05, 2017
Article Accepted on : February 10, 2018
This article describes the study of producing composites based on polyimide matrices containing nanostructured boron carbide as reinforcement particles. To improve the adhesion between ceramic particles and matrix material the boron carbide was pre-modified. The composites were produced using high temperature in situ polymerization method, with aromatic tetracarboxylic dianhydrides and aromatic diamines used as monomers. In particular, 4,4’-isopropylidene diphthalic anhydride, 4,4’-(p-phenylenedioxi)bis[phthalic anhydride], 4,4’-oxydianiline, 4-[4-(4-aminophenoxy)phenoxy]aniline were used as monomers, while the filler content was 20 to 60 wt. %. Mechanical properties, imidization degree and thermal resistance of the composites produced were studied as well.
KEYWORDS:polyimide composite; dianhydride aromatic acids; boron carbide; nanofiller; polyimide; adhesion
Download this article as:| Copy the following to cite this article: Averina E. A, Yegorov A. S, Bogdanovskaya M. V, Wozniak A. I, Zhdanovich O. A. Polyimide Composite Materials Containing Modified Nanostructured Boron Carbide. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Averina E. A, Yegorov A. S, Bogdanovskaya M. V, Wozniak A. I, Zhdanovich O. A. Polyimide Composite Materials Containing Modified Nanostructured Boron Carbide. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44439 |
Introduction
Polymers are known to be prone to substantial destruction during operation in high radiation environment. These structural changes can be prevented by reinforcing a polymer with radiation-absorbing fillers. The variety of polymer and reinforcement materials allows to adjust the strength, stiffness, operating temperature range, radiation protection and other properties in a focused way by selecting ingredients and changing component ratio and composite micro- and nanostructures. Polyimides have the highest thermal resistance and ionizing radiation resistance among polymer matrices, so using these polymers it the most promising in terms of producing radiation protection composites.
Aromatic polymers are known to show generally higher radiation resistance than aliphatic ones, which is due to the energy dissipation caused by aromatic ring’s resonance structures [1-5]. Thus, the subjects of interest are aromatic polymer materials of high radiation resistance, such as epoxy resins [6-8], polyimides [9, 10], phenol resins [11, 12], polystyrene [13,14], polyethers [15] and composites based on this materials.
It is polygeteroarylenes, with the chains formed by continuously interlacing aromatic cycles and heterocycles, that are featured by the highest heat and thermal resistance (Figure 1).
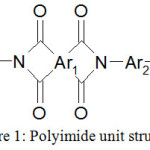 |
Figure 1: Polyimide unit structure Click here to View figure |
In recent decades numerous studies were aimed at modifying molecular interactions in the polyimides to improve their processability using conventional technologies, such as melt casting or solvent casting, while retaining a polymer’s thermal-oxidative resistance.
High thermal resistance (chemical resistance during heating, Td) of polyimides is due to their structure stabilization and bond strengthening caused by conjugation effect, since a cycle’s heteroatom has a lone electron pair (in case of nitrogen) and there are high electronegativity atoms (in case of carboxyl group oxygen), while imide cycle is stabilized due to C-N bond conjugation.
Radiation resistance of polyimides is due to the high bond strength within imide cycle and polyimide exposure accompanied by competing processes, such as macrochain breakdown and molecular cross-linking.
In addition to their exceptionally high radiation resistance, polyimides have a number of valuable and unique physical, mechanical, electrical and chemical properties. They can be used over a long period of time at 200ºС, while their short-term use is possible even at 480ºС. In fact, polyimides have excellent physical and mechanical properties within wide temperature range. These characteristics enable polyimide domination in numerous fields of application.
Polyimides provide basis for the whole conventional lineup of polymer materials, such as coupling and filled compositions, including those containing continuous high-strength and high-modulus fibers and their textile forms; glass, carbon and organic fiber-reinforces plastics; bonding agents, sealants and films).
Using nanosized powder particles of radiation-absorbing materials (BN, B4C, Pb and W) leads to 1.5-time increase in neutron absorptivity and 30-40% increase in gamma-ray scattering factor.
Composition materials were shown to have not only radiation resistance, but also higher mechanical strength and higher thermal resistance, comparing with an unfilled polymer [16]
Experimental Section
Materials and Reagents
Dianhydrides (4,4’-isopropylidene diphthalic anhydride), 4,4’-( p-phenylenedioxi)bis[phthalic anhydride]) and diamines (4,4’-oxydianiline, 4-[4-(4-aminophenoxy)phenoxy]aniline) were synthesized by the authors using developed techniques. M-cresol and benzoic acid were purchased from the Vekton LLC, while boron carbide was purchased from the ImKhimService LLC.
Composite Manufacturing
Nanocomposites were produced using in-situ polymerization method over the filler, i.e. nanostructured boron carbide.
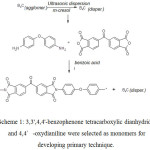 |
Scheme 1: 3,3′,4,4′-benzophenone tetracarboxylic dianhydride and 4,4′-oxydianiline were selected as monomers for developing primary technique.
|
65 ml of m-cresol and 2.00 g of nanostructured B4C (calculated as 20wt.%. of the final polyimide) were fed into 250-ml three-neck flask equipped with a sonicator, reflux condenser and inert gas inlet tube. The reaction mass was subjected to ultrasonic treatment, with treatment duration and acoustic wave frequency values shown in Table 2, followed by mixing with standard mechanical mixer. 3.28 g (16.4 mmol) of 4,4′-oxydianiline was added to the mixture and mixed till completely dissolved (about 10 minutes), followed by single addition of 5.30-g (16.4 mmol) of 3,3′,4,4′-benzophenone tetracarboxylic dianhydride and 3.00 g (24.6 mmol) of benzoic acid. The reaction mass is then mixed at room temperature for 10 minutes, heated to 80ºС and mixed again for several hours, followed by heating to 180ºС and mixing over the duration specified in Table 3. Once the dwelling was over, the reaction mass was cooled down to room temperature and poured into the glass equipped with magnetic mixer, which contained 250-ml ethyl alcohol. The deposit produced was filtered out and flushed with 50-ml ethyl alcohol filter, followed by drying in the drying chamber at 150ºС and15 mm Hg.
The dispersion quality was determined by means of sample microphotograph, which was obtained using scanning electron microscopy and Hitachi SU-1510 VP-SEM, with its voltage of 7 kV and its working distance of 5.5 mm.
Imidization degree was determined to assess the imidization efficiency for Fourier-transform infrared spectra of the polyimide nanocomposites produced. Characteristic signals of polyimide, polyamidoacid and monomer absorption are shown in Table 1 [1]. Imidization degree could be determined by comparing the adsorption band intensity of the sample film (I) and completely imidized reference film (I0) ( Kapton©, manufacturer: DuPont). To make up the film thickness difference, a normalizing factor, i.e. adsorption band intensities, which remain unchanged during imidization (e.g. 1500-cm-1 aromatic adsorption bands (I(rel) и I0(rel))), was used. Imidization degree (%) was determined using the following equation:
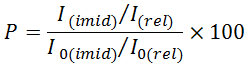
Table 1: Characteristic adsorption frequencies used for determining imidization degree [1]
| Compound | Adsorption band, см-1 | Origin |
| Aromatic imides | 1780 | C=O asymmetric stretching |
| 1720 | C=O symmetric stretching | |
| 1380 | C-N stretching | |
| 725 | C=O bend | |
| Amidoacids | 2900-3200 | COOH and NH2 |
| 1710 | C=O (COOH) | |
| 1660 (amide 1) | C=O (CONH) | |
| 1550 (amide 2) | C-NH | |
| Anhydrides | 1820 | C=O |
| 1780 | C=O | |
| 720 | C=O | |
| Amines | ~3200 (two bands) | NH2symmetric structure |
| NH2asymmetric structure |
Adsorption spectra were recorded using Fourier-transform infrared spectrometer Bruker VERTEX 70 with extended IR range of 8000 to 50 cm-1 and FT-Raman module RAMII, Bruker Optics.
Results and Discussions
Determination of optimal modification mode for nanostructured boron carbide
To produce the composites of high filler content (over 30wt.%.) without significant loss of mechanical properties and loss-of-filler composite cracking, the surface of nanostructured boron carbide needs to be modified.
Organosilicon compounds containing alkoxysilyl groups are the most commonly used modifying agents capable of forming stable bonds with inorganic particles. During the interaction between organosilicon compounds and the surface of boron oxide, which can be found on the surface of nanostructured boron carbide, a chemical bonding between an organosilicon fragment and a particle’s surface hydroxyl groups occurs, eventually leading to the hydrophobization of filler’s particle surface, which allows to form stable bond with a polymer, while reactive amino group appears on particle surfaces, thus allowing to achieve additional covalent bonding.
In this case (3-aminopropyl) triethoxysilane (trade name: silane coupling agent КН-550), which is widely used for composite production, was used as a modifying agent. This cross-linking agent is very sensitive to moisture, so using it for filler surface modification requires dried solvents.
The method of infrared spectroscopy by cross-linking agent’s residual signals following complete filler drying was used for assessing the efficiency of modifying nanostructured boron carbide surface. Modification origin is determined by 3200-2400 cm-1 bands that refer to NH2 group of the cross-linking agent and by up-to-1610 cm-1 signal that refers to CH2 groups of (3-aminopropyl) triethoxysilane.
10 g of nanostructured nonmodified boron carbide powder and 100 ml of dry solvent were fed into 250-ml four-neck flask equipped with a sonicator, reflux condenser, inert gas inlet tube and thermocouple (Table 4). The powder was dispersed in the solvent by using 20 kHz ultrasound for 15 minutes, followed by replacing sonicator waveguide with mechanical mixer and adding (3-aminopropyl) triethoxysilane (Table 4). The reaction mass was then mixed and heated simultaneously (Table 5). Once the dwelling was over, the reaction mass was cooled down to room temperature and poured into the glass, followed by centrifuging and filtering out modified nanostructured boron carbide powder, which was then dried in the drying cabinet at 80ºС for 6 hours.
Table 2: Temperature impact on the efficiency of modifying nanostructured boron carbide surface
| № | Solvent | Dwelling temperature, ºС | Dwelling duration, h | Functional group share of the product, rel. units % |
| 1 | Toluene | 90 | 1 | 98 |
| 2 | 100 | |||
| 3 | 100 | |||
| 95 | 1 | 98 | ||
| 2 | 100 | |||
| 100 | 2 | 100 | ||
| 110 | 2 | 100 | ||
| 2 | o-Xylene | 90 | 1 | 98 |
| 2 | 100 | |||
| 100 | 2 | 100 | ||
| 120 | 2 | 100 | ||
| 140 | 2 | 100 |
As the series of temperature adjustment experiment showed, 2-hour toluene or o-Xylene treatment was enough for surface modification.
Nanostructured boron carbide surface modification
10 g of nanostructured nonmodified boron carbide powder and 100 ml of toluene were fed into 250-ml four-neck flask equipped with a sonicator, reflux condenser, inert gas inlet tube and thermocouple. The powder was dispersed in the solvent by using 20 kHz ultrasound for 15 minutes, followed by replacing sonicator waveguide with mechanical mixer and adding 20 g of (3-aminopropyl) triethoxysilane. The reaction mass was then mixed and heated to 90ºС simultaneously, followed by 2-hour dwelling at this temperature. Once the dwelling was over, the reaction mass was cooled down to room temperature and poured into the glass, followed by centrifuging and filtering out modified nanostructured boron carbide powder, which was then dried in the drying cabinet at 80ºС for 6 hours.
Filler surface modification
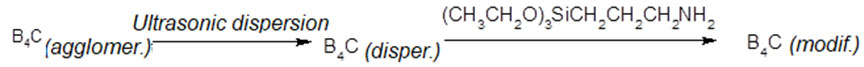
The evenness of filler distribution inside composite material was assessed using scanning electron microscopy and Hitachi SU-1510 VP-SEM, with its voltage of 7 kV and its working distance of 5.5 mm. Figures 2 and 3 show composite microphotographs featured by visible distribution of particular elements. Modifying the filler with (3-aminopropyl) triethoxysilane allows to trace its distribution by that of the silicon. Figure 4 shows IR-spectrum of a composite material based on nanostructured boron carbine and polyimide (BPDA/ODA/20В4С), which was produced using Fourier-transform infrared spectrometer Bruker VERTEX 70 with extended IR range of 8000 to 50 cm-1 and FT-Raman module RAMII, Bruker Optics.
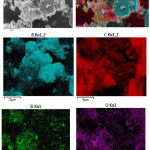 |
Figure 2: Filler distribution inside composite material |
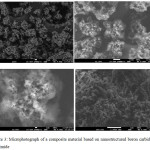 |
Figure 3: Microphotograph of a composite material based on nanostructured boron carbide and polyimide |
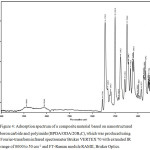 |
Figure 4: Adsorption spectrum of a composite material based on nanostructured boron carbide and polyimide (BPDA/ODA/20В4С), Click here to View figure |
Which was produced using Fourier-transform infrared spectrometer Bruker VERTEX 70 with extended IR range of 8000 to 50 cm-1 and FT-Raman module RAMII, Bruker Optics.
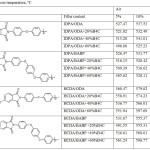 |
Table 3: 5- и 10-% weight loss temperature,ºС Click here to View table |
Conclusion
During the study the authors produced a number of composites based on polyimide matrices containing nanostructured boron carbide as reinforcement particles. The composite materials produced showed high thermal resistance and even filler distribution inside polymer matrix. The composites of high filler content (over 30wt.%.) also showed high thermal resistance due to modification of boron carbide with 3-aminopropyl-triethoxysilane.
The technique developed is suitable for polyimide matrices and various nanostructured boron carbide ingredients inside a composite an ensures even filler distribution and the highest imidization degree.
Acknowledgements
Applied researches are carried out with state financial support represented by the Ministry of Education of Russia under the Agreement on granting subsidies №14.625.21.0035 of October 27, 2015. (Unique identifier of Applied Scientific Researches (project) RFMEFI62515X0035).
References
- Chen J.; Huang X.Y.; Jiang P.K.; Wang G.L. Protection of SEBS/PS blends against gamma radiation by aromatic compounds. J. Appl. Polym. Sci. 2009, 112, 1076-1081.
CrossRef - Elsa R.; Curtis W.F.; James H.O.D.; David J.T.H. Radiation effects on polymeric materials, irradiation of polymeric materials. Am. Chem. Soc. 1993,1-8.
- Bonin H.W.; Bui V.T.; Pak H.; Poirier E.; Harris H. Radiation effects on aluminum-epoxy adhesive joints. J. Appl. Polym. Sci. 1998, 67, 37-47.
CrossRef - Laricheva V.P. Effect of ionizing radiation on epoxy oligomers of different structures and manufacture of new promising materials on their base. Radiat. Phys. Chem, 2008, 77, 29-33.
CrossRef - Rinaldi G.; Maura G. Durable glass-epoxy composites cured at low temperatures – effects of thermal cycling, UV irradiation and wet environment. Polym. Int. 1993, 31, 339-345.
CrossRef - Apel P.Y.; Blonskaya I.V.; Oganessian V.R.; Orelovitch O.L.; Trautmann C. Morphology of latent and etched heavy ion tracks in radiation resistant polymers polyimide and poly(ethylene naphthalate). Nucl. Instrum. Methods B. 2001, 185, 216-221.
CrossRef - Coltman R.R.; Klabunde C.E. x Mechanical strength of low-temperature-irradiated polyimides – a fivefold-to-tenfold improvement in dose-resistance over epoxies. J. Nucl. Mater. 2001, 103, 717-721.
CrossRef - Gilfrich H.P.; Rosinger S.; Wilski H. The radiation-resistance of thermoset plastics. 1. Phenolic plastics with inorganic fillers. Radiat. Phys. Chem, 1991, 38, 431-443.
CrossRef - Gilfrich H.P.; Rosinger S.; Wilski H.; The radiation-resistance of thermoset plastics. 2. Phenolic plastics with organic fillers. Radiat. Phys. Chem. 1991, 38, 573-582.
CrossRef - Spiegelberg S.H.; Cohen R.E.; Argon A.S. Effects of radiation on mechanical- properties of polybutadiene/polystyrene blends. J. Appl. Polym. Sci. 1995, 58, 85-94.
CrossRef - Tagawa S. Pulse-radiolysis and laser photolysis studies on radiation-resistance and sensitivity of polystyrene and related polymers. Radiat. Phys. Chem. 1986, 27, 455-459.
CrossRef - Aliev R.; Navarro-Gonzalez R.; Medina R. A comparative radiation degradation of some aromatic polyesters. Polym. Bull. 2006, 57, 499-504.
CrossRef - Hu H.S.; Wang Q.S.; Qin J.; Wu Y.L.; Zhang T.K.; Xie Z.S.; Jiang X.B.; Zhang G.G.; Xu H.; Zheng X.Y.; Zhang J.; Liu W.H.; Li Z.H.; Zhang B.P.; Li L.B.; Song Z.H.; Ouyang X.P.; Zhu J.; Zhao Y.L.; Mi X.Q.; Dong Z.P.; Li C.; Jiang Z.Y.; Zhan Y.P. Study on composite material for shielding mixed neutron and gamma- rays. IEEE Trans. Nucl. Sci. 2008, 55, 2376-2384.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









