Phytosterol Screening of Skin and Seed Extracts of Wild Grape Ampelocissus Martinii Planch. Fruits
Wilaiwan Simchuer and Prasong Srihanam
Creative Chemistry and Innovation Research Unit, the Center of Excellence in Chemistry, Department of Chemistry, Faculty of Science, Mahasarakham University, Kantarawichai, Maha Sarakham 44150, Thailand.
Corresponding Author E-mail: prasong.s@msu.ac.th
DOI : http://dx.doi.org/10.13005/ojc/340235
Article Received on : October 29, 2017
Article Accepted on : January 05, 2018
Reports on phytosterol in wild grape (Ampelocissus martinii Planch.) fruits are rarely available worldwide. This work investigated the total triterpenoid content (TTC) and total sterol content (TSC) in skin and seed extracts from different growth stages of wild grape fruits using a spectrophotometric assay. In addition, we analyzed some free sterols using high performance liquid chromatography (HPLC). The mature fruit showed the highest content of TTC in both the skin and seed extracts. The TSC content was found the highest from skin extract in the ripe stage. The two most common phytosterols found were stigmasterol and β-sitosterol. The stigmasterol content was found the highest in the skin extract of the immature, while β-sitosterol was found in the seed extract of the ripe. This work indicated that the skin and seeds of wild grape fruits might be new sources of phytosterol.
KEYWORDS:Wild Grape; Phytochemical; Phytosterol; Spectrophotometric Assay
Download this article as:| Copy the following to cite this article: Simchuer W, Srihanam P. Phytosterol Screening of Skin and Seed Extracts of Wild Grape Ampelocissus Martinii Planch. Fruits. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Simchuer W, Srihanam P. Phytosterol Screening of Skin and Seed Extracts of Wild Grape Ampelocissus Martinii Planch. Fruits. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=43936 |
Introduction
Phytochemicals are secondary metabolites that are non-nutritive, naturally occurring, and biologically active compounds and found in the plant kingdom1. Phytochemicals enrich the biological activity potential, including anti-inflammation, anti-cancer, antimicrobial, and antioxidant. There are more than a thousand known phytochemicals2. Phytosterols are one group of triterpenoid and pervasive in plants. Generally, their structures look like to cholesterol, but side chain has differed in the alkyl position. Among more than 200 different compounds of phytosterol, β-sitosterol, stigmasterol, and campesterol are predominate types in higher plants and human diets. The several of vegetable oil, nuts, fruit, berries, grapes, and cereals are important sources of the plant sterols. Wild grape is a potent source of antioxidants and anti-bacterials from their polyphenol compounds: flavonoids and phenolic acids3,4. According to the phytosterol structures, they should be reduced low-density lipoprotein (LDL)-cholesterol levels in body. Cholesterol was reduced of approximately 10%by consumption phytosterol of 2 g/day5. Ruggiro and coworkers 6 found that the contents of phytosterols were varied in different tissues as well as phenological stage. Millan and coworkers7 isolated and evaluated the phytosterol content from cultivated grape and wine by modified a liquid chromatography and ion-trap mass spectrometer. The results found that the highest content of phytosterol was in the grape seed. Wild grape (Ampelocissus martinii Planch.) is a tropical fruit in the Vitaceae family. It is a traditional herbal ingredient and has been used in folk medicine in Thailand, in which the root is more popular than other plant parts. The shoots and their immature fruit are used as food in Thailand. The stem and fruit of wild grape are similar to cultivated grape with regards to the color and stage of fruit development. With previous works, we found that the wild grape fruits composed of various phytochemicals and each have good biological activities, especially antioxidant and antibacterial activities3,4,8. However, information about phytosterol in wild grape has never been reported.
In this study, we focused on screening phytosterol in the skin and seed of different growth stages of wild grape fruit extracts. Quantification of the total triterpenoid content and total sterol content by spectrophotometric assay was performed. Finally, some free sterols without derivatization step were also studied using HPLC.
Materials and Methods
Chemicals for Analysis
β-sitosterol, stigmasterol, ergosterol, and cholesterol standards were purchased from Across Organics (New Jersey, USA). The organic solvents, methanol, ethanol, hexane isopropanol, percholic acid, ursoilc acid, and hydrochloric acid, were obtained from Sigma-Aldrich (USA). Other chemicals, acetic acid, sulfuric acid, vanillin, and potassium hydroxide were purchased from Sigma-Aldrich (USA). The HPLC grade of acetonitrile and methanol were used as mobile phase (Merck, Germany). The other chemicals as well as solvents of analytical grade used were not further purifying.
Sample Preparation
Raw materials of wild grape fruits were collected from Roi-Et Province, Northeastern Thailand from August to September 2016 and categorized by their fruit colors into immature (green), mature (red), and ripe (black). The skin and seeds of fruits were separated and processed to dry by oven at 60˚C for 8 h. The dried samples were prepared into a fine powder by a mortar and kept in sealed plastic bags until further analysis.
Extraction of Sample
The samples of wild grape were extracted with an orbital shaker by modification of the Hara and Radin method9. Samples were mixed with 150 mL of n-hexane:IPA (3:2 v/v). The extract was filtered via filter paper (Whatman No. 1). The debris was re-extracted twice with 150 mL of the same solvent and then pooled together with the first time extraction. To obtain the biphasic system, 200 mL of 6.67 % Na2SO4 in water was added to the combined filtered solution. After gentle mixing, the mixture was allowed at 4˚C to give phase separation. The lipid appeared in upper organic phase was then dried by evaporation and kept under nitrogen in the dark at -20˚C until use.
Investigation of Total Triterpenoid Content (TTC)
The TTC was investigated using the Nimethod10 with some modifications. Briefly, 0.3 mL of each extract were contained in a tube and heated at 100˚C in a water bath to dryness. Then added of 0.05 mL vanillin-acetic acid solution (5:95, w/v) and 0.8 mL perchloric acid and immersed in a water bath at 60˚C for 15 min before moving into an ice-water bath. Finally, the prepared solution was mixed with 5 mL acetic acid and left at room temperature about 15 min. The mixture solution and blank as a reference was measured their absorbance by a spectrophotometer at 548 nm. Results are expressed as mg ursolic acid equivalent (UrE)/100 g DW.
Investigation of Total Sterol Content (TSC)
The TSC was investigated followed by the Liebermann-Burchard (LB) colorimetric method11 with minor modifications. Cholesterol was used as the standard. Firstly, 50 µL concentrated H2SO4 was mixed with 2 mL acetic anhydride to obtain the LB color reagent. Then, 1 mL of sample extract in chloroform was added to the LB color reagent, stirred for 1 min, and kept at room temperature for 13 min. The mixture solution was measured absorbance using a spectrophotometer at 650 nm. Results are expressed as mg cholesterol equivalent (ChE)/100 g DW.
Analysis of Sterol Using High Performance Liquid Chromatography (HPLC)
Sample Treatment
Firstly, the organic phase extracts and then evaporated until dryness. The dryness was added with 5 mL of water to reconstitute solution before adding 5 mL of 37% HCl with heating at 80˚C for 2 h. The solution was shaken vigorously every 30 min. The samples were stand at room temperature to cool. After that, the solution was mixed with 10 mL chloroform-methanol (1:1, v/v) for free sterol extraction. To separate the solution layer, the solution was vortex-mixed and centrifuged at 3000 rpm for 5 min at 4˚C. Each organic at lower layer was collected and taken for evaporation under a gentle stream of nitrogen to dryness. The dry sample was dissolved in water and mixed with ethanolic solution in 2 M KOH at 80˚C for 1 h for sponification. After cooling, the extracts were then secondary extracted by the same solvent and procedure to obtain the lower organic layer again. They were then dried and reconstituted in 250 µL of acetonitrile. Before analysis by HPLC, the extracts were centrifuged to receive solution without contaminant.
Instrumental Condition
Chromatographic separations were performed on an HPLC using the reverse-phase with a C18 column (2.1 mm x 250 mm, 5 µm). The acetonitrile and water mixture system was used as mobile phase with the isocratic mode of flow rate. The column temperature was adjusted at 35˚C during the whole run and used 20 µL injection volume. The diode array detector was operated at 205 nm.
Statistical Analysis
The data were obtained from triplicate and revealed as means ± SD. The SPSS ver. 19 for Windows was used for data analysis. The means significances were compared at p <0.05 using one-way analysis of variance.
Results and Discussion
Total Triterpenoid Content
The mature seed showed a higher total triterpenoid content than the other tissues (Fig. 1). There was 1.392±0.157 mgUrE/100gDW, which indicates a significant difference when compared with the immature and ripe stages. The result indicates that the ripe skin extract showed the lowest total triterpenoid content of 0.852±0.009 mgUrE/100 gDW.
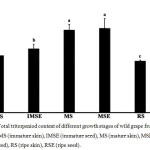 |
Figure 1: Total triterpeniod content of different growth stages of wild grape fruits extracts; IMS (immature skin), IMSE (immature seed), MS (mature skin), MSE (mature seed), RS (ripe skin), RSE (ripe seed). Click here to View figure |
Different letters indicated significantly different at p <0.05 of means.
Figure 1 indicates that the triterpenoid content of wild grape fruit alters during fruit development; with an early deposition of high levels that subsequently decreases during ripening as this stage has the termination of oleanolic acid biosynthesis that may result in the modulation of the mechanical toughness of the cuticle that typically appears in numerous fruit [12]. Indeed, triterpenoids are another group of plant secondary metabolites, and the pharmaceutical and physiological activities of triterpenoids are diverse and important and include antidiabetic, anti-HIV, and antioxidant properties [13]. Triterpenoids contained triterpene acids, such as ursolic acid, oleanolic acid, and triterpene alcohol [12]. Therefore, this plant could be important pharmaceutically due to its total terpenoid contents.
Total Sterol Content
The total sterol contents of different growth stages of wild grape fruits’ skin and seed extracts are presented in Figure 2. The result indicated that the highest total sterol levels were obtained from the ripe skin (109.483±3.836 mg ChE/100 gDW), mature seed (99.702±1.562 mg ChE/100 gDW), and immature skin (71.485±1.367 mg ChE/100 gDW). The lowest total sterol level was found in the mature skin (23.923±7.894 mg ChE/100 gDW). It can be observed that the skin of the immature and ripe stages had higher total sterol contents than the seed tissue, while the skin of the mature stage had a lower content than the seed. This report contrasts with the phytosterol content found in cultivar grapes. The skins of the grape had decreased phytosterols levels in the last stage of maturity or during the transition from pre-véraison to véraison [12],[14]. It is well known that phytosterols are involved the function of cell membrane including membrane fluidity and permeability regulations and membrane-associated metabolic processes [15]. The excessive cell division and differentiation in the early stage of ripening is a main reason on biosynthesis of compounds such as phytosterol for controlling membrane fluidity [16]. Moreover, sterol biosynthesis was stopped in the last ripening stage due to the end of enzymatic activity, and those of phytosterols were changed to other sterol forms or to stanol [14]
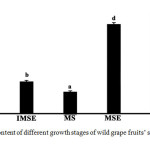 |
Figure 2: Total sterol content of different growth stages of wild grape fruits’ skin and seed extracts. Click here to View figure |
Different letters indicate means significantly different at p <0.05.
Contents of free sterol
Free phytosterols such as stigmasterol and β-sitosterol, were analyzed using the HPLC technique without derivatization step. This method was simple and avoided thermal conditions, like the GC method, so analyzed samples were not degraded or oxidized to other forms. The reliability of the present study was confirmed by the data shown in Table 1. The coefficient of determination values of sterol were in the range 0.9989-1.000. Moreover, the recovery values were in the range 84-114%. Figure 3 shows the chromatogram of the sterol standard and crude extract of different wild grape tissues. The chromatograms of all wild grape extracts did not have peak nos. 1 and 2, which were ergosterol and cholesterol. This suggested that ergosterol can be classified as a yeast sterol, while cholesterol is an animal sterol [16]. The contents of free sterol; stigma sterol and β-sitosterol, are shown in Table 2. β-sitosterol was a main compound found in all wild grape stages. High β-sitosterol contents found in the mature seed, immature skin, and ripe skin were 198.418, 171.538±0.001, and 162.095±0.001 mg/100gDW, respectively. The results showed that all extracts could be found the sterol. Exactly, the sterol is indispensable compounds in plasma membranes structure. Moreover, sterols are converted to steroidal hormones in eukaryotes, mammals, insects and higher plants17.
Table 1: HPLC data of sterol standards.
| Sterol | Calibration curve | Determination coefficient (R2) | LOD (mg/100g) | LOQ (mg/100g) | % recovery |
| Ergosterol | y=9885.7x+11961 | 0.9989 | 0.031 | 0.105 | 114.102 ± 0.015 |
| Cholesterol | y=8636.2x+907.33 | 0.9990 | 0.027 | 0.091 | 96.798 ± 0.001 |
| Stigmasterol | y=10184x+1239.5 | 1.0000 | 0.016 | 0.052 | 84.000 ± 0.003 |
| β-sitosterol | y=6513x-1988.7 | 0.9993 | 0.026 | 0.085 | 97.625 ± 0.002 |
Table 2: Composition and sterol content of wild grape extracts analyzed by HPLC.
|
Stigmasterol β-sitosterol |
||||||
|
mg/100gDW |
SD |
%CV |
mg/100gDW |
SD |
%CV |
|
|
Immature (skin) |
135.409a |
0.001 |
0 |
171.538a |
0.001 |
0.001 |
|
Immature (seed) |
3.809b |
0 |
0.007 |
46.475b |
0 |
0.001 |
|
Mature (skin) |
20.856c |
0.003 |
0.013 |
79.460c |
0.002 |
0.003 |
|
Mature (seed) |
69.462d |
0.001 |
0.001 |
198.418d |
0 |
0 |
|
Ripe (skin) |
22.636e |
0 |
0.002 |
162.095e |
0.001 |
0 |
|
Ripe (seed) |
4.256f |
0 |
0.007 |
14.353f |
0 |
0.002 |
Different letters in the same column represent significant differences at p < 0.05 of means.
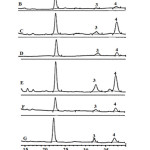 |
Figure 3: HPLC chromatogram of sterol standard and wild grape extracts detected by DAD at 205 nm. Click here to View figure |
The letter on each chromatogram represents sterol standard (A), IMS (B), IMSE (C), MS (D), MSE (E), RS (F), and RSE (G). The number on the top peak represents ergosterol (1), cholesterol (2), stigmasterol (3), and β-sitosterol (4).
Conclusion
The mature stages of both wild grape skin and seed accumulated high total triterpenoid contents. When considering the skin extract, the total sterol contents had rapidly decreased by the mature stage and increased again by the end of the ripening stage. This is in contrast to the accumulation of sterol in the seed tissue, which increased rapidly by the mature stage and had decreased rapidly by the end of the ripening stage. This is the first report of the quantification of phytosterol from wild grape (Ampelocissus martinii Planch), which gave different results from previous report of cultivated grape. This work indicated that the skin and seeds of wild grape fruits might be important sources of phytosterol and should be further studied to obtain more information, especially biological activities.
Acknowledgenents
The authors would like to thank the Faculty of Science and Mahasarakham University, Thailand for financial support of this work. Moreover, we would like to thank the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education, Thailand for partial financial support.
References
- Jong, A.; Plat, J.; Mensink, R. P. J. Nutr. Biochem. 2003, 14, 362.
CrossRef - Cai, R.; Hettiarachchy, N. S.; Jalaluddin, M. J. Agric. Food. Chem. 2003, 51, 1623.
CrossRef - Boonsod, Y.; Sangdee, A.; Srihanam, P. Br. J. Pharm. Res. 2014, 4, 23.
CrossRef - Jirum, J.; Sangdee, A.; Srihanam, P. Int. J. Res. Ayurveda Pharm. 2013, 4, 337.
CrossRef - Bard, J. M.; Paillard, F.; Lecerf, J. M. Diabetes Metab. 2015, 41, 69.
CrossRef - Ruggiro, A.; Vitalini, S.; Burlini, N.; Bernasconi, S.; Iriti, M. Food Chem. 2013, 41, 3473.
CrossRef - Millan, L.; Sampedro, M. C.; Sanchez, A.; Goicolea, M. A. J. Food Comp. Anal. 2015, 42, 171.
CrossRef - Wongnarat, C.; Srihanam, P. Int. J. Appl. Chem. 2016, 12, 337.
- Carreno, M.M.; Knol, D.; Janssen, H. G. J. Chromatogr. A. 2016, 1428, 316.
CrossRef - Ni, Q. X.; Xu, G. Z.; Wang, Z. Q.; Gao, Q.X.; Wang, S.; Zhang, Y.Z. Int. J. Mol. Sci. 2012, 13, 2249.
CrossRef - Kim, E. Clin. Chem. 1969, 15, 1171.
- Pensec, F.; Paczkowski, C.; Grabarczyk, M.; Wozniak, A.; Melanie, B. G.; Bertsch, C.; Chong, J.; Szakiel, A. J. Agric. Food Chem. 2014, 62, 7998.
CrossRef - Beieth, A.; Santos, A. R.; Calixto, J. B.; Hess, S. C.; Messma, I.; Ferrari, F.; Yunes, R. A.; Panta Med. 1999, 65, 50.
CrossRef - Le Fur, Y.; Hory, C.; Bard, M. H.; Olsson, A. Vitis. 1994, 33, 127.
- Trautwein, E. A.; Demonty, I. DOSSIER, OCL. 2007, 14, 259.
CrossRef - Bouali, I.; Trabelsi, H.; Herchi, W.; Martine, L.; Albouchi, A.; Bouzaien, G.; Sifi. S.; Boukhchina, S.; Berdeaux, O. Food Chem. 2014, 164, 309.
CrossRef - Ohyama, K.; Suzuki, M.; Kikuchi, J.; Saito, K.; Muranaka, T. Proc. Natl. Acad. Sci. USA. 2009, 106, 725.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









