Novel Organotin(IV) Complexes Derived from Chiral Benzimidazoles: Synthesis, Molecular Structure and Spectral Properties
A. Akremi1 , A. Noubigh2 and M. J. A. Abualreish3
, A. Noubigh2 and M. J. A. Abualreish3
1,2,3Department of Chemistry, Faculty of Sciences, Northern Border University, Arar 91431, Kingdom of Saudi Arabia.
1Department of Chemistry, Faculty of science, El Manar University, Tunis, Tunisia.
2Laboratory of Physical Chemistry of Materials , Preparatory Institute for Scientific and Technical Studies of La Marsa, 2070, Carthage University, Tunisia.
3Department of Chemistry ; Faculty of Science and Technology; Omdurman Islamic University; Sudan.Corresponding Author E-mail: akrimimi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340220
Article Received on : November 10, 2017
Article Accepted on : January 10, 2018
The synthesis of homoleptic five-coordinate heteroaryl tin(IV) compounds using three chiral N-heterocyclic substituents, 2-(1-aminoethyl) benzimidazole (1), 2-(pyrrolidin-2-yl) benzimidazole (2) and 2-(1-amino-2-mercaptopropylyl) benzimidazole (3) ligands is described. It has been found that ligands 1 and 2 behave in the bidentate N,N donor fashion, however, ligand 3 behave in the bidentate N,S donor. Overall ligands furnished, with moderate yields, their corresponding complexes [(CH3)2Sn(C9H10N3)Cl] (4),[(CH3)2Sn(C9H12N3)Cl] (5) and [(CH3)2Sn(C9H10N3S)Cl] (6). The characterization of all compounds by various spectroscopic methods UV-Vis, FT-IR, NMR, RX and elemental analyze provided insights into the diversity of complex formation.
KEYWORDS:Five-Coordinate; Heteroaryl Tin(IV); Chiral Benzimidazoles; Bidentate; Moderate Yields
Download this article as:| Copy the following to cite this article: Akremi A, Noubigh A, Abualreish M. J. A. Novel Organotin(IV) Complexes Derived from Chiral Benzimidazoles: Synthesis, Molecular Structure and Spectral Properties. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Akremi A, Noubigh A, Abualreish M. J. A. Novel Organotin(IV) Complexes Derived from Chiral Benzimidazoles: Synthesis, Molecular Structure and Spectral Properties. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44375 |
Introduction
The organotin(IV) compounds have been widely investigated during the last few decades due to its multifaceted picture compromising a broad spectrum of biocidal properties, catalytic activity, use as stabilizers and appealing structural diversity [1-11].
Structural studies have always been prominent in organotin chemistry, and particularly the structural changes which occur between the solution and solid states. The tin atom in the complexes can be penta-coordinated [12-14] or hexa-coordinated [15-18] and the complexes often show fluxional stereochemical behavior [16,19].
On the other hand, benzimidazoles are known to exhibit a wide range of pharmacological properties including antiviral and antibacterial activities [20-23]. Another feature of the benzimidazole ligands is functionalization at the non-coordinated nitrogen of the benzimidazole moiety, which provides a mode for modification of their properties.
In addition, chiral organometallic complexes exhibit various pharmacological properties. The binding of chiral drugs to DNA usually shows stereoselectivity, which affect their pharmacokinetic profiles and biochemical activities [24-26].
The present work describes the synthesis and characterization of three tin(IV) compounds derived from chiral bidentate ligands 2-(1-aminoethyl)benzimidazole (1), 2-(pyrrolidin-2-yl)benzimidazole (2) and tridentate ligand 2-(1-amino-2-mercaptopropylyl)benzimidazole (3) (Scheme 1).
Results and Discussions
2-Alkylminobenzimidazoles (ABzH) 1-3 have been synthesized from reaction of o-phenylenediamine with L(+)-alanine, L(+)-proline and L(+)-cysteine respectively (Scheme 1) [27].
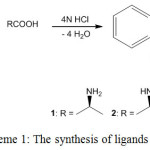 |
Scheme 1: The synthesis of ligands 1-3. Click here to View scheme |
Organotin complexes (Me2SnABzCl) 4-6 were prepared by reaction of 2-Alkylminobenzimidazole derivatives (ABzH) 1-3 with Me2SnCl2 in aqueous alkaline medium at room temperature (Scheme 2). The composition of isolated complexes was confirmed by the findings obtained from elemental analyses. It has been found that in all AbzH 1-3, one chloride was substituted by monoanionic ligands and yielded their corresponding mononuclear complexes. Thus, ligands (1) and (2) behave in the Bidentate N,N donor fashion, however, ligand 3 behave in the bidentate N,S donor. This result may be explained by the considerable simultaneous acidic and nucleophile behave of sulfur in mercaptan group [29] (Table 1). Characterization of synthesized complexes and their nature of bonding were examined by spectroscopic investigations.
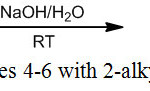 |
Scheme 2: Synthesis of complexes 4-6 with 2-alkylamino benzimidazoles (ABzH). |
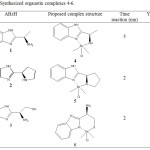 |
Table 1: Synthesized organotin complexes 4-6. Click here to View table |
FT-IR
All ligands (ABzH) 1-3 display broad medium stretching vibrational band (n(N—H)) at 3450 cm-1, which does not change on complexation [28]. However, as well used as hydrochloride benzimidazoles, the broad bands at 3150-2400 cm-1 corresponding to n(N+—H) stretching vibration disappear after reaction to create new bands at 500-600 cm-1 related to Sn—C stretching vibration. Thus, a medium or relatively weak band at 1625 cm-1 with a small shoulder at 1522 cm-1 due to stretching vibration of C=N, were considerably become larger and sharper (1633 and 1507 cm-1) confirming the complexation with tin atom in all complexes 4-6 [28]. Moreover, the medium intensity band at 2525 cm−1, assigned to the ν(S–H) vibration in the free ligand 3, has not been observed in the spectra of the corresponding complex (6), while, a short band has been appeared in the 421 cm−1 range assigned to the ν(Sn–S) (Fig. 1). The above discussed data are in agreement with the deprotonation of the thiolic group after coordination on the tin center [29].
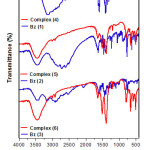 |
Figure 1: Infra-red spectra of synthesized compounds. |
UV-Vis Property
The solutions of complexes 4-6 were prepared with DMSO at room temperature, and their concentration was 1×10-5 mol/L. The spectrum showed the general appearance of absorption maxima for 2-alkylamino benzimidazole moiety [30] (Fig. 2).
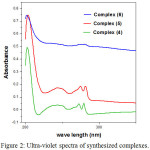 |
Figure 2: Ultra-violet spectra of synthesized complexes. |
NMR Spectroscopy
1H NMR Spectroscopy
The 1H NMR spectra of tin complexes were measured in DMSO-d6 solution and exhibited signals with chemical shift values in accordance with their proposed structures. All complexes 4-6 spectra showed signals at 10.8-10.5, 7.6-7.1, 4.31-4.62, and 1.50 which were assigned to the NH proton, chiral CH, and the CH3-Sn [31], correspondingly. The latter signals confirm simultaneously the presence of the tryptophan moiety in the complexes 4-6 [32], and the non-coordination of –NH– group of tryptophan moiety on the tin atom [28]. In addition, the ligands (1) and (3) revealed NH2 proton signal at 1.61-1.76 ppm [33] which has disappeared in the complexes (4) and (5) respectively, indicating coordination of the –NH2 group on the tin atom. Moreover, the ligand (2) showed NH (pyrrolidine group) proton signal at 2.16 [34] which has disappeared in the complex (5) suggesting probably coordination of –NH– moiety to the atom center. In complex (5), the 1H NMR signals at 1.80-2.40, 3.09-3.30, and 4.64 ppm indicates the presence of pyrrolidine group [34]. Furthermore in complex (6), cysteine moiety was confirmed by the presence of signals at 2.57 and 4.08 ppm [33]. Thus, the appearance of signals at 1.63-1.78 indicates the presence of –NH2 group which confirms coordination of the deprotonated thiolate group on the tin center instead of the latter amino group [29].
119Sn NMR Spectroscopy
To investigate the ligand-binding modes to the tin atom in solution [35], 119Sn NMR spectra was measured. It is generally recognized that 119Sn chemical shifts are very sensitive either to the coordination modes and ligand types [36]. Thus, a range of d values from +200 to –60 have been reported for four-coordinated, –90 to –190 for five-coordinated and –210 to –400 for six-coordinated diorganotin(IV) complexes in solution [37]. The chemical shift values for the synthesized compounds 4-6 are –186, –204, and –207 ppm, respectively confirming the penta-coordination mode.
XRD Analysis
The XRD powder diffraction spectra of synthesized complexes were recorded with a scan rate about 0.02º min-1 and range 2θ = 5-55 degree (Fig.3). The registered spectra revealed peak with maxima at 2θ = 12.48-12.42, d = 7.23-7.05 Ǻ, and FWHM = 0.2503-0.2435 (Fig.4). All data are summarized in table 2. The obtained results demonstrate closer parameters regarding to the first (maxima) peaks of XRD spectra for all complexes.
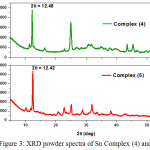 |
Figure 3: XRD powder spectra of Sn Complex (4) and (5). |
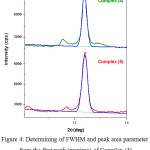 |
Figure 4: Determining of FWHM and peak area parameter from the first peak (maxima) of Complex (4) and (5) by Origin software. |
Table 2: First peak (maxima) parameters of XRD spectra.
| First peak (maxima) parameters | ||||
| FWHM | Peak area | 2θ | d-spacing [Å]* | |
| Complex 4 | 0.2435 | 1883.7 | 12.48 | 7.23 |
| Complex 5 | 0.2503 | 2289.1 | 12.42 | 7.10 |
| Complex 6 | 0.2488 | 2161.4 | 12.45 | 7.05 |
(*) d-spacing [Å] values were obtained by X’pert High Score software.
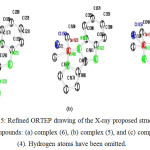 |
Figure 5: Refined ORTEP drawing of the X-ray proposed structure of compounds: (a) complex (6), (b) complex (5), and (c) complex (4). Hydrogen atoms have been omitted. |
Calorimetric Measurements
The differential scanning calorimetry measurements of synthesized complexes were recorded on heating between 200 and 800 K with a scanning rate 5 K min-1 (Fig.6). The first thermogram showed the combustion of organic part of complex (4) which located at 592 K. The values of enthalpy and entropy were resulted from the peak area are as follow: ∆H = -774.8 kJ/mol and ∆S = -1.5 kJ mol-1 K-1.
The second thermogram revealed the combustion of complex (5), which was achieved in three steps; at 473, 568, and 638 K, correspondently. The values of enthalpy and entropy were determinate from the peak area are as follow: ∆H = -668 kJ/mol and ∆S = -1.2 kJ mol-1 K-1.
The third thermogram displayed the combustion of complex (6), which was completed in three steps located at 555 K, 588 K, and 652 K, respectively. The values of enthalpy and entropy were obtained from the peak area are as follow: ∆H = -664 kJ/mol and ∆S = -1.3 kJ mol-1 K-1.
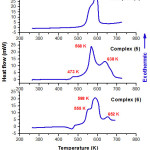 |
Figure 6: DSC measurements of complexes 4-6 |
Experimental
Apparatus and Materials
The melting point of complexes was recorded by a differential scanning calorimetric instrument (DSC 131 evo–Setaram, France). The IR spectra were measured using a Nicolet iS5 Infrared Spectrometer in KBr (Thermo Scientific, USA).). 1H-NMR, 13C-NMR and 119Sn spectra were recorded by Bruker Avance 300 spectrometer (Bruker, France) at 300 and 75 MHZ, respectively. Chemical shifts were reported in ppm from internal TMS for 1H, 13C and 119Sn. Elemental analysis was obtained by means an Exeter Analytical CE–440 Elemental instrument (Exeter Analytical, UK). X-ray spectra were recorded by an Oxford X-calibur Gemini diffractometer (Santa Clara, California, USA) equipped with EOS CCD detector using monochromated Mo Ka radiation (k = 0.71073 A˚) at room temperature. Dimethyltin dichloride, o-phenylenediamine, L(+)-alanine, L(+)-proline, and L(+)-cysteine were purchased from Sigma-Aldrich and used as received.
Typical Procedure for the Synthesis of Complexes 4-6
ABzH 1-3 (5 mmol) were taken in 10 mL aqueous solution of NaOH (5 mmol) and stirred at room temperature for 20 minutes. After that, an aqueous solution of dimethyltin dichloride (5.5 mmol) was added dropwise with continuous stirring. After couple of minutes stirring, a pale yellow powder was precipitated, which was filtered out, washed simultaneously with water and ethanol and recrystallized from a mixture ethanol/DMSO (95/5).
[(CH3)2Sn(C9H10N3)Cl] (4)
M.p. 480-482 K; Calc. for C11H16ClN3Sn (%): C, 38.36; H, 4.68; N, 12.20. Found: C, 38.34; H, 4.66; N, 12.18; Selected IR data (KBr) [ʋ/cm-1]: 3449 ʋ(NH); 1633 and 1507 ʋ(C=N); 780 ʋ(Ar); 576 ʋ(Sn-C); 500 ʋ(Sn-N);. UV-vis (EtOH) [λmax/nm]: 202, 272, 279. 1H NMR (DMSO-d6, ppm): 1.5 (CH3-Sn) 4.3 (-CH); 7.6-7.2 (Harom); 10.8 (BzNH) . 13C NMR (DMSO-d6, ppm): 151 (C=N); 114-128 (Carom); 46 (CH); 22 (-CH3), 8.1 (CH3-Sn). 119Sn NMR (DMSO-d6, ppm): -186.
[(CH3)2Sn(C9H12N3)Cl] (5)
M.p. 453-455 K; Calc. for C13H18ClN3Sn (%): C, 42.15; H, 4.90; N, 11.34. Found: C, 42.14; H, 4.88; N, 12.32; Selected IR data (KBr) [ʋ/cm-1]: 3451 ʋ(NH); 1637 and 1507 ʋ(C=N); 792 ʋ(Ar); 579 ʋ(Sn-C); 500 ʋ(Sn-N). UV-vis (EtOH) [λmax/nm]: 203, 274, 281. 1H NMR (DMSO-d6, ppm): 1.5 (CH3-Sn); 1.4-2.4 (m, -CH2-CH2-); 3.1-3.3 (CH-NH-CH2-); 4.6 (-CH); 7.1-7.6 (Harom), 10.5 (BzNH). 13C NMR (DMSO-d6, ppm): 154 (C=N); 115-129 (Carom); 46 (CH); 21 (-CH3); 8.4 (CH3-Sn). 119Sn NMR (DMSO-d6, ppm): -204.
[(CH3)2Sn(C9H10N3S)Cl] (6)
M.p. 485-487 K; C11H16ClN3SSn (%): C, 35.09; H, 4.28; N, 11.16. Found: C, 35.08; H, 4.26; N, 11.15; Selected IR data (KBr) [ʋ/cm-1]: 3450 ʋ(NH); 1636 and 1507 ʋ(C=N); 788 ʋ(Ar); 576 ʋ(Sn-C); 501 ʋ(Sn-N). UV-vis (EtOH) [λmax/nm]: 201, 273, 280. 1H NMR (DMSO-d6, ppm): 1.4 (CH3-Sn) 2.6 (CH2-SH) 4.0 (-CH); 7.1-7.6 (Harom), 10.6 (BzNH). 13C NMR (DMSO-d6, ppm): 154 (C=N); 117-130 (Carom); 44 (CH); 23 (-CH3); 8.2 (CH3-Sn). 119Sn NMR (DMSO-d6, ppm): -207.
Conclusion
Chiral penta-coordinate organotin(IV) complexes were successfully prepared and characterized using different analytical techniques. The obtained spectroscopic data were accordant with the proposed structures of products. The complexes (4) and (5) were resulted via N,N coordination to tin atom by their analogous ligands (1) and (2), respectively, whereas, the complex (6) was achieved by N,S chelation of tin center. The XRD spectra of compounds revealed closer parameters about the first (maxima) peak. DSC studies showed that the decomposition of the complexes proceeded via exothermic combustion.
Acknowledgment
The authors wish to acknowledge the approval and the support of this research study by the grant N°. SCI-2016-1-6-F-4679 from the Deanship of Scientific Research in Northern Border University, Arar, KSA.
References
- Xiao, X.; Liang, J.; Xie, J.; Liu, X.; Zhu, D.; Dong, Y. J. Mol. Struct. 2017, 1146, 15, 233-241.
CrossRef - Basu Baul, T. S.; Kehie, P.; Duthie, A.; Guchhait, N.; Raviprakash, N.; Mokhamatam, R. B.; Manna, S. K; Armata, N.; Scopelliti, M.; Wang, R.; Englert, U. J. Inorg. Biochem. 2017, 168, 76-89
CrossRef - Javed, F.; Sirajuddin, M.; Ali, S.; Khalid, N.; Tahir, M. N.; Shah, N. A.; Rasheed. Z.; Khan, M. R. Polyhedron 2016, 104, 80-90.
CrossRef - Saeed, A.; Channar, P. A.; Larik, F. A.; Jabeen, F.; Muqadar, U.; Saeed, S.; Flörke, U.; Ismail, H.; Dilshad, E.; Mirza, B. Inorg. Chim. Acta 2017, 464, 204-213.
CrossRef - Niu, L.; Li, Y.; Li, Q. Inorg. Chim. Acta Part B 2014, 423, 2-13.
CrossRef - Tariq, M.; Muhammad, N.; Sirajuddin, M.; Ali, S.; Shah, N. A.; Khalid, N.; Tahir, M. N.; Khan, M. R. J. Organomet. Chem. 2013, 723, 79-89.
CrossRef - El-Khalafy, S. H.; Hassanein, M. T.; Etaiw, S. E. H.; Badr El-Din, A. S. Arab. J. Chem. 2017, 10, 2, S2829-S2835.
CrossRef - Etaiw, S. E. H.; El-bendary, M. M.; Appl. Catal., B: Environmental 2012, 126, 326-333.
CrossRef - Henno P. van Dokkum, Sherri L. Huwer, Regul. Toxicol. Pharm. 2005, 41, 1, 73-81.
CrossRef - Flick, E. W. Plastics Additives (Third Edition) 2002, 10-23.
CrossRef - Davies, A. G. Organotin Chemistry (second edition), (2004).
CrossRef - Teo, S.-B.; Teoh, S.-G.; Okechukwu, R. C.; Fun, H.-K. Polyhedron 1994, 13, 2223-2228.
CrossRef - Vasnin, S.V.; Cetrullo, J.; Geanangel, R. A.; Beranl, J. Inorg. Chem. 1990, 29, 885-888.
CrossRef - Pettinari C.; Marchetti, F.; Pellei, M.; Cingolani, A.; Barba, L.; Casetta, A. J. Organomet. Chem. 1996, 515, 119-130.
CrossRef - Pettinari, C.; Pellei, M.; Marchetti, F.; Santini, C.; Miliani, M. Polyhedron 1998, 17, 561-576.
CrossRef - Pettinari, C.; Marchetti, F.; Cingolani, A. Polyhedron 1996, 15, 1263-1276.
CrossRef - Lobbia, G. G.; Bonati, F.; Ceechi, P.; Leonesi, D. J. Organomet. Chem. 1990, 391, 155-163.
CrossRef - Das, V. G. K.; Keong, Y. C.; Wei, C.; Smith, P. J.; Mak, T. C. W. J. Chem. Soc., Dalton Trans. 1987, 129-137.
CrossRef - Davies, A. G. and Smith, P. J. Chap. 11 in G. Wilkinson, Stone, F. G. A. and Abel, E. W. (eds) Comprehensive Organometallic Chemistry, Pergamon Press, Oxford 1982, 2, 519-627.
- Dittmer, A.; Woskobojnik, I.; Adfeldt, R.; Drach, J. C.; Townsend, L. B.; Voigt, S.; Bogner, E. Antiviral Research 2017, 137, 102-107
CrossRef - Tonelli, M.; Novelli, F.; Tasso, B.; Vazzana, I.; Sparatore, A.; Boido, V.; Sparatore, F.; La Colla, P.; Sanna, G.; Giliberti, G.; Busonera, B.; Farci, P.; Ibba, C.; Loddo, R. Bioorg. Med. Chem. 2014, 22, 4893-4909.
CrossRef - Shahini, C. R.; Achar, G.; Budagumpi, S.; Tacke, M.; Patil, S. A. Inorg. Chim. Acta, 2017, 466, 432-441.
CrossRef - Moreira, J. B.; Mann, J.; Neidle, S.; McHugh, T. D.; Taylor, P. W. Int. J. Antimicrob. Agents 2013, 42, 4, 361-366.
CrossRef - (a) Stinson, S.C.; Chem. Eng. News 2001, 79, 45-56;
CrossRef
(b) Xiaogang, Q.; Trent, J. O.; Fokt, I.; Priebe, W.; Chaires, J. B. Proc. Natl. Acad. Sci. U.S.A 2000, 97, 12032-12037.
CrossRef - Miao, T.-F.; Xu, L.-C.; Miao, Q.-Q.; Wang, Q.-Q., Wang, N.-L. Inorg. Chim. Acta 2015, 43474-43478.
- Zhou, X.-Q.; Li, Y.; Zhang, D.-Y.; Nie, Y.; Li, Z.-J.; Gu, W.; Liu, X.; Tian, J.-L.; Yan, S.-P. Eur. J. Med. Chem. 2016, 114, 244-256
CrossRef - Phillips, M. J. Chem. Soc. C., 1928, 2393.
CrossRef - Tavman, A.; Ülküseven, B. Trans. Met. Chem. 2000, 25, 324-328.
CrossRef - (a) Pellerito, L.; Prinzivalli, C.; Casella, G.; Fiore, T.; Pellerito, O.; Giuliano, M.; Scopelliti, M.; Pellerito C. J. Inorg. Biochem. 2010, 104, 750-758.
CrossRef
(b) Domazetis, G.; Magee, R. J.; James, B. D. J. organometal. Chem. 1978, 162, 239. - Steck, E. A.; Nachod, F. C.; Ewing, G. W.; Gorman, N. H. J. Am. Chem. Soc. 1948, 70, 10, 3406-3410.
CrossRef - Sedaghat, T.; Goodarzi, K. Phosphorus. Sulfur. Silicon Relat. Elem. 2007, 182, 2227-2233.
CrossRef - (a) Alatorre‐Santamaria, S.; Gotor Fernández, V. Eur. J. Org. Chem. 2009, 15, 2533-2538;
CrossRef
(b) Li, Y.; Ding, K.; Sandoval, C.A. Org. Lett. 2009, 11, 907-910.
CrossRef - (a) Chen, R.-H.; Xiong, J.-F.; Peng, P.; Mo, G.-Z.; Tang, X.-S.; Wang, Z.-Y.; Wang, X.-F. Asian J. Chem. 2014, 26, 3, 926-932;
(b) Peng, P.; Xiong, J. F.; Mo, G. Z.; Zheng, J. L.; Chen, R. H.; Chen, X. Y.; Wang, Z. Y. Amino Acids 2014, 46, 2427-2433.
CrossRef - Reddy, K. R.; Krishna, G. G.; Rajasekhar, C. V. Synth. Commun. 2007, 37, 4289-4299.
CrossRef - Pettinari, C. in: J.C. Lindon (Ed.), Heteronuclear NMR Applications (Ge, Sn, Pb), Encyclopedia of Spectroscopy and Spectrometry, Academic Press, London, 1999.
- Damude, L. C.; Dean, P. A. W.; Masivannman, V.; Srivastava, R. S.; Vitali, J. J. Can. J. Chem. 1990, 68, 1323-1331.
CrossRef - Holeček, J., Nádvorník, M.; Handlíř, K.; Lyčka, A. J. Organomet. Chem. 1986 315, 299-309.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









