Lithium Including Mixed Sodium Inside Graphene Oxide (GO) as Anodic Electrodes for Ion Batteries
Samira Bagheri1, Majid Monajjemi2 , Alireza Ziglari1 and Afshin Taghva Manesh1
, Alireza Ziglari1 and Afshin Taghva Manesh1
1Department of Chemistry, College of science, Central Tehran Branch, Islamic Azad University, Tehran, Iran.
2Department of Chemical Engineering, Central Tehran Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: m_monajjemi@srbiau.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/340248
Article Received on : October 21, 2017
Article Accepted on : January 20, 2018
Graphene oxide has a great potential as a suitable material for anodic sodium ion battery (NIBs) due to its unique behavior. The calculated reversible of lithium ion capacity of GO/ Li &Na /GO based anodic materials are widely improved comparing to the conventional graphite-based anodic materials. By this investigation GO sheets have been localized into the graphene as an item for enhancing electrochemical& Physical ratio. Moreover, the structure of GO/Li& Na/GO can be used for improving the capacities and electronics transport in the GO sheets-based NIBs. Therefore, these modifications of GO sheet and designing of GO/Li&Na/GO structures provide a strategy for increasing the performance of GO-based anode. GO/Li&Na/GO could also be accomplished into the free- standing anodes without any of binders or current’s collectors, which will leads to increased energies densities of overall battery’s designing.
KEYWORDS:Graphene Oxide (GO); Anodic Materials; Lithium Ion Batteries
Download this article as:| Copy the following to cite this article: Bagheri S, Monajjemi M, Ziglari A, Manesh A. T. Lithium Including Mixed Sodium Inside Graphene Oxide (GO) as Anodic Electrodes for Ion Batteries. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Bagheri S, Monajjemi M, Ziglari A, Manesh A. T. Lithium Including Mixed Sodium Inside Graphene Oxide (GO) as Anodic Electrodes for Ion Batteries. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44209 |
Introduction
Sodium-ion battery is a type of re-chargeable battery which uses sodium-ions as its charge carriers and is very similar to LIBTs in many ways. Na ions are 33% larger in diameter and 2.1 times heavier within lower gravimetric capacities than Li-ion batteries1. In addition Na metals are more active than Li with the standard electrode potentials which is ~0.3V higher than Li.
By the Sony it has been realized a commercialization of xC6/Li 1-x CoO2 cells1 reaction and mechanism in 1991. LIBs are representative energies storage devices based on electrochemical energies, widely used in small grid storage systems. Within discovery of high quality reversible, low-voltage1 Li-intercalations carbonaceous1 material, the suitable electronic and chemical performances of LIBs concerning energies and power densities1, as well as the progress in the cell designing and manufacturing, has been made LIBs extremely successful for mobile equipment.
Although; the Na+ cation has a greater radius than the Li+ cation, that causes a few of the superior LIB anode’s materials un-suitable for [NIBs]2, concerns regarding2 the extra availability of lithium resources2 is raising. A Sodium2 ion battery has drawn increasing attention3 due to Na is an mineral abundant3 element and shares2 common properties within Li2-4. Specially, graphite5 is used6 for anodic electrodes in the present commercial7 LIBs, while those have been reported for having a very low capacity6 of when used as an anodic electrode for NIBs5-7.
Both disordering carbons and Nano-flakes8 (CNFs) exhibited a high Na intercalation9 capacities and emerges8 as the leading candidates for NIB applications8,9 . In this study we tried to build a model for GO for investigating the mechanisms for Na intercalation9 into the layered domains9. Although, the mechanism7 of Na+ cation inserting into disturbed carbons are still controversial10. It is confirmed that a bigger interlayer distances11 of those carbons, which are bigger than interlayers distances of graphite, help Na+ ion intercalation. Consequently, the defects might be enhanced the Na+ intercalations through the strong bonding energies for overcoming the van der Waals energies between graphene sheets12. In this study it has been evaluated the reliabilities of several semi-empirical corrections and vdW exchange’s correlation functional for determining the optimal12 methods of this work. Graphited electrodes are currently the most general materials used for the anodes of commercial12 batteries due to its capabilities for reversible12 lithium intercalation13 in a layered crystal that represent maximum theoretical Sodium’s storage capacity13.
NIBs includes of a positive electrode(+) and a negative electrode(-) with suitable conducting electrolytes where store14 electric energies as the forming of Na-intercalations compounds11. Electrodes12, separator13, and electrolytes are the major components14 in the NIB batteries which the anodic electrodes play important behavior in the mechanism of those kind devices12,13.
The structures and properties14 of graphitic oxide depends on a synthesis method or degree of oxidation13. In addition graphite, typically preserves14 the layer structures of those parent graphite, but the layers are buckled14 and the interlayer’s spacing14 is about two times bigger than the graphite that provides suitable environment for sodium diffusion in a NIBs11
The detailed15 structures are still not described because of the strong disordering and irregular13 packing of those layers12. GO layer is about 1.1 ± 0.2 nm thick10,11. STM spectroscopy indicates the presences of some regions14 where the oxygen atoms are arranged12 in a rectangular13 pattern within lattice constant14 0.26 nm × 0.42 nm11 the edges15 of one layer is terminated with COOH and CO groups10. GO exfoliates15 and decomposes16 when heated (rapidly) at moderately14 high temperatures within the formation14 of finely dispersed13 amorphous carbons, somewhat similar for activating carbon12.
XRD14, FTIR15, Raman16, XPS15, AFM14, TEM13, etc. are some general techniques for characterizing the GO samples13. Since the distributions of oxygen’s functionalities on GO sheets are poly-disperse fractionations methods using emulsion16 stabilizations can be used for characterizing and separating “GO” on the basis of oxidation14. During of the charging step, the “NIBs” Sodium ions released15 from the cathodes electrodes to move inside the electrolyte and is inserted16 into the anodes. Upon15 discharging, Sodium ions are extracted15 from the anode electrodes and move back to the cathode14. Although the electrolytes establishes15 high ionic conductivities between anodes and cathodes, this electrolyte is not responsible15 for the conduction16 of those electrons16 and the half of reaction, so will move through the extra14 external wires.
Several experiments16 have been performed for confirming the utilization of graphene nano-sheets16 and nano-ribbons17 for enhancing lithium capacity to improve the recharge cyclic performances. Furthermore semi-empirical15 and molecular17 orbital calculations have been used for investigating sodium ion storage states among the graphene sheets15, as well as some heteroatom-substituted carbon materials16. There are several studies on anodic materials17-20 and most of them focus on carbons and inorganic materials18.
Discharging and charging of LIBTs in graphitized19 carbon is well investigated up to now21-24. It has also been established how the repulsive19 interaction can result in the pure stages during intercalation22. Although efforts have been tried for finding suitable replacements23, now days only the carbonaceous goods are used for the commercial anodes25. Carbonaceous properties widely depends on the preparing materials such as precursor or heat treatment26
By this work, charging or dis-charging of Na-cations have been investigated24 in Graphite oxide (GO) with the (+) electrode reaction25 as: NaCoO2 ⇄ Na1-x CoO2 + x Na+ + xē and the (-) electrode mechanism as: xGO+ x Na+ + xē ⇄ x NaGO, while the whole reaction is: NaCoO2 + x GO ⇄ Na1-xCoO2+ x Na GO. It has been predicted that a sodium atom is stored26 through two mechanisms: intercalation26 and alloying27. GO similar Graphite is applied as small band gap semi-metals because of its excellent conduction treatment under the influence27 of electrical fields28, 29. Interlayer forces30 are nothing, and the distances among GO layers31 are large allowing29 Na-ions for diffusing between GO sheets22. Electrical conductivity of the Na-GO increases, Because of the electron donor nature of the Na33. Based on some previous studies we have used several methods to simulate the NIBs and calculated the NMR, solvent effect24, voltages, charges and physical properties of our model32-43and77-81 .Typical commercially31 used sodium -ion battery including several interconnected32 electrochemical cells, where each one consist of a GO anode, a cathode formed30 by sodium metal oxide (NaCoO2) and electrolyte same as NaPF6 embedded in a separator felt29.
Na+ Diffusion
Diffusion of Na+ in the cells determine the key performance31 of Na-ion batteries cells, with the charge and discharge rates, practical and cycling capacities and stabilities respectively. The governing45 equations describing44 the diffusion44 process are famous as Fick’s law as:
![]()
which “ji” is an ion in flux condition, molcm−2 s−1, Di are diffusivities of solute (i=1,2), cm2 s−1and Ci are concentrations of species i, (molcm3)44. The proportionalities44 factors D are the diffusivities or diffusion coefficients as
![]()
In condensed systems both liquid & solid, diffusion45 is governed45 by random particles, leads to a position exchanges by its neighbor. The kinetic parameter of these processes are temperatures depend to an Arrhenius types relationships
![]()
In liquid phase, the temperature related of diffusion45 is less than the solid form. There are no successful44 first-principle calculations which have been made, because of in-sufficient interpretation of the liquid structure44 . Therefore, an easy expression44 is gotten from Stoke’s drag law which is used as an alternative45 for a diffusivities expression in the liquids55 frequently (Eq. 3). Na+ is quite big comparing to electrons46; the radius of a Na-ion is larger than that of an electron (electron: 10−22 m)46. The motions of Na-ions are strongly impeded through the ions as discussed below47.
Cathode Materials
In an extended approach they can be classified as 1- Layered compounds NaMO2 (M = Co, Ni, Mn), 2- Spinel compounds NaM2O4 (M = Mn, etc.) 3– Olivine compounds NaMPO4 (M = Fe, Mn, Ni, Co, etc.). When a Na-ion diffuses56 out of the cathode during the charges cycles the valence states of the transition46 metal ion change as; the Fe2+ to Fe3+. The reaction in cathode can be written as: (Fig.1)
NaFePO4 – xNa+ – x e– ⇄ x NaPO4 +(1-x) NaPO4 and Fe2+– e– ⇄ Fe3+
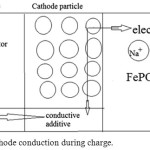 |
Figure 1: The cathode conduction during charge. |
Anode Materials
In the anode, Na+ are found to be the suitable electropositive particles with large reversible capacities. However, due to safety considerations51, metallic Na has been substituted by various carbonaceous materials such as GO. GO-sodium anode has much lower gravimetric and volumetric energies density than pure sodium which leads toward the development of 3d transition metal oxides51. (MxOy, M = Fe, Co, Ni, Mn, Cu). These materials are able to incorporate more than one Na+ per metal through conversion reactions giving higher capacities in comparison to carbon anodes51. During discharge, Na+ ion is extracted52 from the internal layered of graphite, those passes through the electrolyte63 and intercalated between the NaCoO2 compounds Figure.2.
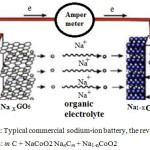 |
Figure 2a: Typical commercial sodium-ion battery, the reversible process is: m C + NaCoO2 NanCm + Na1-nCoO2 |
Carbon-sodium anodes have much lower gravimetric and volumetric energy densities than pure sodium which lead towards the development of interstitial-free 3d transition metal oxides
Electron Density Profiles
It has been defined53 as
![]()
Where ηi is occupation64 orbitals (i), φ is the wave functions of the orbitals, χ and C are basis function and coefficient matrix respectively. Atomic units can be explicitly rewritten as follows (e/Bohr3).
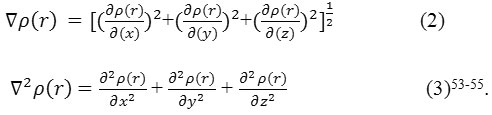
Electron localization64 and chemical reactivity has been described by Bader66.
Hamiltonian kinetic energy density K(r)
It is defined, since the expected values of kinetic energies operator
![]()
can be rewritten by definitions53. One of commonly used definition55 is:
![]()
“G(r)” is a positive definition of kinetic energy densities.
![]()
K(r) and G(r) are related to Laplacian electrons density55
![]()
ELF or Electronic localization function
Becke and Edgecombe57 exhibited that spherically averages like-spin pair probability56,57 are localization functions
![]()
Where
![]()
and
![]()
For close-shell system, since
![]()
D and D0 terms can be simplified as
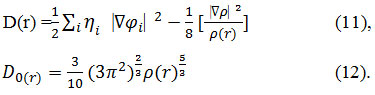
Savin et al. have reinterpreted67,68 ELF is a meaningful68,69 for Kohn-Sham69,70 DFT or even post-HF wave-function58.
LOL is the other function in tight localization regions68 same as ELF, Schmider&Becke paper59,60.
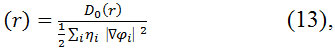
Wher
![]()
Do (r) for spin-polarized and close-shell systems as the same way as in ELF61.
Local Information Entropy
This entropy is defined as exclusive spaces through minimizing informationentropy62 by Parr et al63,64.The formula of Shannon’s information64 entropies for normalized and continuous probabilities function is
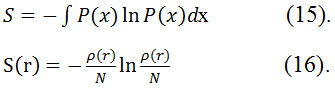
Electrostatic potential (ESP)
The total electrostatic potential measures the set of functions as GIPF75 which for the molecular surfaces and macro properties65, 66. There are a lot of reviews on ESP, interested readers are suggested to consult66, 67.
It is accurate in normal cases. Reduced -density -gradient or RDG and Sign (l2)*r are a couple parameter for revealing of the weak interaction region68. The basic applications are exemplified in Sections 4.100.1 and 4.200.1. RDG is defined as
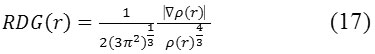
By default x is 0.05, it can be nullify this treatment by setting the parameter to zero.
![]()
is an averaged electron density of atom A.
Computational Details
Calculations are performed using both Gaussian and GAMESS-US packages68. Pm6, Extended-Huckel and Pm3MM including pseudo=lanl2 calculations69.
M06 and m06-L (DFT) functional is based on pseudo-potentials69-70. The (PBE)71 exchange-correlations functional are made via minimization of the whole energy69-71. We employed DFT with the van der Waals densities for modeling the van der Waals capacitor72 in the medium-range73 interactions such as the interactions74 of two cylinders. In some further calculation the interaction energy between x sodium and GO sheets. The interaction energy was calculated via the Mp6 method in all items according to
ΔEs (eV) = { Etotal _ (ExNa + EGOsheets )} + EBSSE
Where the “ΔEs” is the stability energy of system73.
Result and Discussion
The Laplacian of electron density via Eqs (7, 8) for the Sodium diffused in the GO system which are arranged and listed in table.1. We have used
Table 1: Density, energies, G(r), K(r)and U(r) for 7 sodium insertion
ρs (r) = ρα (r) – ρβ (r)
then the polarization-spin valuable will be replaced instead of spin densities
![]()
ELFs, Localized locators (LOLs) and Local Entropies including ESP charges, potential energies, of two graphene layers (GO sheets) have been listed in tables1-2 and these data have been plotted in seven figures figs.1-7.The values of ξ yielded from zero to the local region of un-polarized cases, are shown as a complete polarized situation and are listed in (Table1).
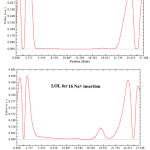 |
Figure 3: ELF, LOL, K(r) and G( r) for 16 Na+ insertion between two layers of GO sheets.
|
 |
Figure 4: LOL for various insertion of Na+ between two layers of GO |
Kintic energies, Lagrangien kintic energies densities, and the electrostatic potentials can be calculated as eqs. (9), (10) and:
![]()
where RA and ZA denotes vectors charges of atom A, and are listed in tables 1,2.
If pseudo-potentials are used, Z is shown the explicitly expression of those electrons. Z can be stand for the atomic charges (the fourth column), at this item Vnu is useful for analyzing the differences between exact electrostatic potential and the electrostatic potential reproduced by atomic charges. Notice that at nuclear positions, this function will be infinite and may cause some numerical problems in program; hence at these cases this function always returns 1000 instead of infinity.
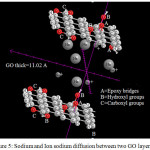 |
Figure 5: Sodium and Ion sodium diffusion between two GO layers |
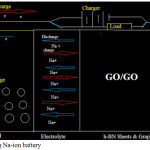 |
Figure 6: Operating Na-ion battery Click here to View figure |
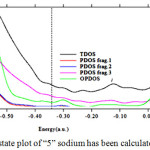 |
Figure 7: Density of state plot of “5” sodium has been calculated of diffusion inside GO |
In which the actual kinetic energy term in D (r) from eqs. 15-16 is replaced by Kirzhnits type gradient expansions, which is
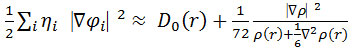
so that ELF is the kinetic energy term.
In this work we have calculated the local information entropies for each Sodium atom via eqs. 19-20 and the integrating of this function over total space yields the entropy. The calculated data for local entropy are listed in Tables 2-5
Table 1: Density, energies, G(r), K(r)and U(r) for 7 sodium insertion
|
Na |
Alpha(α) |
Beta(β) |
G(r) |
K(r) |
U(r) |
|
Na(1) |
0.4604895515E-06 |
0.4604895515E-06 |
0.5744381519E-05 |
– 0.3117752101E-06 |
– 0.5432606309E-05 |
|
Na(2) |
0.2096714075E-05 |
0.2096714075E-05 |
0.1167764372E-04 |
0.3597110963E-06 |
-0.1203735482E-04 |
|
Na(3) |
0.3170935798E-05 |
0.3170935798E-05 |
0.2997362700E-04 |
-0.4869104958E-05 |
-0.2510452204E-04 |
|
Na(4) |
0.2724083172E-05 |
0.2724083172E-05 |
0.5506029806E-04 |
-0.4894711288E-05 |
-0.5016558677E-04 |
|
Na(5) |
0.3552291679E-06 |
0.3552291679E-06 |
0.9933364631E-05 |
-0.1527079624E-05 |
-0.8406285007E-05 |
|
Na(6) |
0.1939734653E-05 |
0.1939734653E-05 |
0.1510844208E-04 |
-0.9991542452E-06 |
-0.1410928784E-04 |
|
Na(7) |
0.3182517515E-05 |
0.3182517515E-05 |
0.6263968474E-04 |
-0.1207120164E-04 |
-0.5056848310E-04 |
Table 2: Electron localization function (ELF), Localized orbital locator (LOL) and RDG for 7 sodium insertion
|
Na |
H(r) |
(ELF) |
(LOL) |
(RDG) |
|
Na(1) |
0317752101E-06 |
02781474745E-09 |
0.4357352365E-04 |
0.4198893007E+02 |
|
Na(2) |
-0.3597110963E-06 |
0.3494449162E-07 |
0.2680478226E-03 |
0.3073178053E+02 |
|
Na(3) |
0.4869104958E-05 |
0.583654322E-07 |
0.2080980347E-03 |
0.3688949858E+02 |
|
Na(4) |
0.4894711288E-05 |
0.194139806E-07 |
0.8795808397E-04 |
0.6126518415E+02 |
|
Na(5) |
0.1527079624E-05 |
0.1182183375E-09 |
0.1635044084E-04 |
0.1358456203E+03 |
|
Na(6) |
0.9991542452E-06 |
0.2729617300E-07 |
0.1819939949E-03 |
0.4296852960E+02 |
|
Na(7) |
0.1207120164E-04 |
0.2114921285E-07 |
0.1001944131E-03 |
0.5308390511E+02 |
Weak interactions (eqs 20-21) have significant influences for conformational structures of the macromolecules, however reproduction by the electron density via abinitio.
As sodium have unpaired electrons, leading to a difference in spin-up &down, when two Sodium atoms are adsorbed.
As a result, spin polarized cluster for NIBTs have a gap which size depends on adsorbed spin polarization. And this system can be replaced with LIBTs completely with high performance.
Conclusion
In conclusion, our calculations have been designed to study the Na adsorption on graphene oxide with two layers of GO. Our results exhibit that adsorption in GO is much stronger than pristine graphene.
Additionally, it has been found the structure of GO can be to improve the electrical transport in NIBs. Therefore, the modification and design of GO structure provide strategies for improving the performance of GO-based anodes. With the increase in defect density by GO sheets, maximum capacities obtained are much higher than that of graphite.
References
- Z. G. Yang, J. L. Zhang, M .C .W. Kintner-Meyer, X. C. Lu, D. W. Choi, J. P. Lemmon, and J. Liu, Chem. Rev. (2011), 111, 3577–3613.
CrossRef - P. Barpanda, G. Oyama, S.-I. Nishimura, S.-C. Chung and A. Yamada, Nat. Commun. , 2014, 5, 4358.
CrossRef - M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater. , 2013, 23, 947.
CrossRef - N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem.Rev. , 2014, 114, 11636.
CrossRef - P. Ge and M. Fouletier, Solid State Ionics, 1988, 28, 1172.
CrossRef - D. Stevens and J. Dahn, J. Electrochem. Soc., 2001, 148, A803.
CrossRef - Y. Mizutani, T. Abe, M. Inaba and Z. Ogumi, Synth. Met., 2001, 125, 153.
CrossRef - D. Stevens and J. Dahn, J. Electrochem. Soc., 2001, 148, A803.
CrossRef - D. Stevens and J. Dahn, J. Electrochem. Soc., 2000, 147, 1271.
CrossRef - Schniepp, H. C.; Li, J. L.; McAllister, M. J.; Sai, H.; Herrera-Alonso, M.; Adamson, D. H.; Prud’Homme, R. K.; Car, R.; Saville, D. A.; Aksay, I. A. The Journal of Physical Chemistry, (2006) B 110 (17): 8535–8539.
- Pandey, D.; Reifenberger, R.; Piner, R.. Surface Science, (2008), 602 (9): 1607.
CrossRef
- Talyzin, A. V.; Szabó, T. S.; DéKáNy, I.; Langenhorst, F.; Sokolov, P. S.; Solozhenko, V. L. The Journal of Physical Chemistry C (2009). ,113 (26): 11279.
CrossRef
- M. Daniela C, K. Dmitry V, B. Jacob M,S. Alexander, S. Zhengzong, S. Alexander, A. Lawrence B, L. Wei, T. James. ACS Nano, (2010), 4 (8): 4806–4814.
- Kumar, Harish V.; Woltornist, Steven J.; Adamson, Douglas H. Carbon.2015.10.083.
- T. Suzuki, T. Hasegawa, S. R. Mukai, and H. Tamon, Carbon,2003, 41:1933-1939
CrossRef - T. Hasegawa, T. Suzuki, S. R. Mukai, and H. Tamon, Carbon, 2004, 42:2195-2200.
CrossRef - M. Noel, and V. Suryanarayanan, Journal of Power Sources , 2002, 111, 193–209.
CrossRef - J. L. Tirado, Materials Science and Engineering R , 2003, 40, 103–136.
CrossRef - L.J. Fu, H. Liu, C. Li, Y.P. Wu, E. Rahm, R. Holze, and H.Q. Wu, 2006, Solid State Sciences, 8 113–128.
CrossRef - E. Frackowiak, and F. Béguin, Carbon , 2002, 40, 1775–1787.
CrossRef - M. S. Whittingham, and A.J. Jacobson (Eds.), Intercalation Chemistry, Academic Press (1982).
- S.A. Safran, and D.R. Hamann, Physical Review Letters. 1979, 42 (21), 1410–1413.
CrossRef - M.D. Levi, D. Aurbach, J. Maier, Journal of Electroanalytical Chemistry 624, (2008) 251–261.
CrossRef - H. Zabel, S. A. Solin (Eds.), Graphite Intercalation Compound I, Springer-Verlag, (1990).
CrossRef - Y.P. Wu, E. Rahm, and R. Holze, Journal of Power Sources , 2003, 114, 228–236.
CrossRef - J.K. Lee, K.W. An, J.B. Ju, B.W. Cho, W.I. Cho, D. Park, and K.S. Yun, Carbon , 2001,39, 1299–1305.
CrossRef - C. De las Casas, and W.Z. Li, J. Power Sources, 2012, 208, 74–85.
CrossRef - K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V.
- Grigorieva, and A.A. Firsov, Science 2004, 306 , 666–669.
- B. Partoens, and F.M. Peeters, Physical Review B , (2006), 74, 075404-1–075404-11
CrossRef - Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem C. 2010, 114, 15315
CrossRef - Monajjemi, M. Struct Chem. 2012, 23,551–580
CrossRef - Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
CrossRef - Monajjemi, M. Chemical Physics. 2013, 425, 29-45
CrossRef - Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics. 2014, 433, 1-11
- Tahan, A. Acta Biotheor, 2011, 59, 291–312
CrossRef - Mollaamin, F.; Monajjemi, M. Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
CrossRef - Monajjemi, M. Theor Chem Acc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
CrossRef - Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
CrossRef - Monajjemi, M. Biophysical Chemistry. 2015 207,114 –127
CrossRef - Physics and Chemistry of Liquids. 2011, 49 (5), 561-571
CrossRef - Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015, 54, 8, 08510
- Majid Monajjemi *, Samira Bagheri, Matin S. Moosavi, Nahid Moradiyeh, Mina Zakeri, Naime Attarikhasraghi, Nastaran Saghayimarouf, Ghorban Niyatzadeh, Marzie Shekarkhand, Mohammad S. Khalilimofrad, Hashem Ahmadin and Maryam Ahadi, Molecules 2015, 20, 21636–21657; doi:10.3390/molecules201219769
CrossRef - Monajjemi, M, Journal of Molecular Liquids, 2017, 230, 461–472
CrossRef - D.S. Wilkinson, Mass Transport in Solid and Fluids, Cambridge University Press, (2000).
CrossRef - H. Mehrer, Diffusion in Solids, Springer, 2007, pp. 27–36.
CrossRef - D.A. Porter, K.E. Easterling, Phase Transformations in Metals and Alloys, 2nd edition, Chapman & Hall, 1992, pp. 1–109 .
CrossRef - R.H. Petrucci, W.S. Harwood, General Chemistry, 7th edition, Prentice-Hall, 1997, pp. 315–343.
- H. Dehmelt, Physica Scripta 1998, T22 102–110.
- C. Wang, and J. Hong, Electrochemical and Solid-State Letters,2007, 10 (3) A65–A69.
- R.A. Huggins, in: C.A.C. Sequeira, A. Hooper (Eds.), Proceedings of the NATO Advanced Study Institute on Solid State Batteries, Alcabideche, Portugal, September 2–17, (1984).
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, and L.J.M. Tarascon, Nature, 2000, 407, (6803), 496-499 .
CrossRef - A.V. Churikov, N.A. Gridina, N.V. Churikova, in: V. Igor, Barsukov, S.Christopher, Johnson, E. Joseph, Doninger, Z. Vyacheslav, Barsukov (Eds.), New Carbon Based Materials for Electrochemical Energy Storage Systems, Springer,2006, pp. 269–276.
- T. Lu, F. Chen, Acta Chim. Sinica, 2011, 69, 2393-2406.
- T. Lu, F. Chen, J. Mol. Graph. Model , 2012,(38): 314-323.
CrossRef - T. Lu, F.Chen Multiwfn: A Multifunctional Wave-function Analyzer, J. Comp. Chem, 2012, (33) 580-592.
- R.F.W. Bader, atoms in Molecule: A quantum Theory (Oxford Univ. press, Oxford, (1990).
- Becke and Edgecombe J. Chem. Phys,1990, 92, 5397.
- A. Savin, Angew. Chem. Int. Ed.Engl,1992, 31, 187.
CrossRef - V.G. Tsirelson, Yu.A. Abramov. Chem. Phys. Lett, 2002, 351, 142–148.
CrossRef - H.L. Schmider ,A.D. Becke J Mol Struct ,Theochem,2000, 527:51 50 .
- Jacobsen Can. J. Chem., (2008), 86(7), 695.
- C. Aslangul, R. Constanciel, R. Daudel and P. Kottis. Adv. Quantum Chem ,1972, 6,93.
CrossRef
- R.G.Parr, P.W.Ayers, R.F.Nalewajski J Phys Chem A,2005, 109:3957–3959.
CrossRef - S. Noorizadeh and E. Shakerzadeh , Phys. Chem. Chem. Phys.2010, 12, 4742.
CrossRef - J.S.Murray, and P. Politzer, J. Mol. Struct. (Theochem),1994, 307, 55.
CrossRef - J. S. Murray Comput. Mol. Sci., 2011, 1, 153.
CrossRef - P. Politzer, J. S. Murray, Theor. Chem. Acc. 2002, 108 134 .
- M.W. Schmidt, K.K.Baldridge, J.A.Boatz, S. T. Elbert, M. S. Gordon, J . H. Jensen, S. Koseki, N. Matsunaga, and K. A. Nguyen et al 2004,14(11) 1347–1363.
- Yan Zhao, Donald G. Truhlar, Theor Chem Account , 2008,120 215–241 .
CrossRef - W. Kohn, L. J. Sham, Phys. Rev. 1965, 140 A 1133-1138 .
- J.P. Perdew, K.Burke , Ernzerhof, Phys. Rev. Lett. ,1996, 77, 3865-3868.
CrossRef - D. L. Klein, R. Roth, A. K. L. Lim, A. P. Alivisatos, and P. L. McEuen, Nature, 1997, (London) 389-699 .
- Yan Zhao, Donald G. Truhlar, Accounts of Chemical Research, 2008, 41(2) 157-167.
CrossRef - B.H. Besler, K.M. Merz, P.A. Kollman, J. comp. Chem. 1990, 11 431.
- L.E. Chirlian, M. M. Francl, J.comp.chem.,1987, 8 894.
- Brneman GM, Wiberg KB J. Comp Chem 1990, (11): 361
- Jalilian, H.; Monajjemi, M.; Aghaei, H, ORIENTAL JOURNAL OF CHEMISTRY, 2017, 33, (5) 2179-2187
- Raoufi, F.; Aghaie, H.; ORIENTAL JOURNAL OF CHEMISTRY, 2017, 33, 5, 2542-2550
- Mohammadian ,N.; Zare, K.; Monajjemi, M.; ORIENTAL JOURNAL OF CHEMISTRY, 2017, 33, No. 4,1595-1602
- Chitsazan,A.; Monajjemi, M.; Aghaei, H.; Sayadian, M.; ORIENTAL JOURNAL OF CHEMISTRY, 2017, 33, No. (3): 1366-1374
- Moosavi, M.S.; Monajjemi, M.; Zare, K.; ORIENTAL JOURNAL OF CHEMISTRY, 2017, 33, No. 4, 1648-1658

This work is licensed under a Creative Commons Attribution 4.0 International License.









