Laccase Biosensor: Green Technique for Quantification of Phenols in Wastewater (A Review)
Yashas S. R , Shivakumara B. P, Udayashankara T. H and Krishna B. M.
, Shivakumara B. P, Udayashankara T. H and Krishna B. M.
Department of Environmental Engineering, JSS Science and Technology University, Mysuru-570006, Karnataka, India.
Corresponding Author Email: sryashas999@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340204
Article Received on : January 28, 2018
Article Accepted on : February 28, 2018
Biosensor; Immobilization; Laccase; Phenol; Sensitivity
Download this article as:| Copy the following to cite this article: Yashas S. R, Shivakumara B. P, Udayashankara T. H, Krishna B. M. Laccase Biosensor: Green Technique for Quantification of Phenols in Wastewater (A Review). Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Yashas S. R, Shivakumara B. P, Udayashankara T. H, Krishna B. M. Laccase Biosensor: Green Technique for Quantification of Phenols in Wastewater (A Review). Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44705 |
Introduction
Quantification of biological or biochemical elements is of utmost importance for environmental monitoring of pollutants like phenols and its derivatives. The ubiquitous presence of phenols and phenolic compounds in the water and wastewater in hundreds of forms and its derivatives has motivated to research on its occurrence, toxicity, quantification, fate, and transport1. Phenolic pollutants join natural water streams along with industrial effluents of chemical-related sector, such as coal refineries, pharmaceutical manufacturing, production of resins, paints, wood processing, textiles, petrochemicals, and pulp, including the manufacturing of phenol1. European Commission (EC) and the United States Environmental Protection Agency (US-EPA) have listed many phenolic compounds as prioritized hazardous pollutants for their monitoring in drinking or natural waters due to their toxicity, carcinogenicity and hazardous nature upon exposure to animals and humans. Permissible concentration limit in natural waters is 0.001 mg/L2 as prescribed by European Commission (EC). The Central Pollution Control Board (CPCB) of India has implemented threshold of 1.0 mg/L of a phenolic compound as the standard for discharge of water to inland surfaces under The Environment (Protection) Rules, 1986. Conventionally spectrophotometric and chromatographic methods are the most common for quantification of phenolic compounds in water and wastewater samples with great accuracy up to nanograms per liter of samples. However, present research in monitoring and quantifying techniques is mainly focused on bio-analytical tools, such as biosensors, which offer advantages over classical ones in terms of selectivity, sensitivity, lesser assay time, non-toxic reagents and reduced cost of analysis3. The reagent-less continuous online analysis is also one of the potential advantages of this device4. Laccase-based biosensors have interesting potential uses in the detection of phenolic compounds in the food industry and wastewaters as well as in biomedical and bioremediation applications5. Usually, classical analytical methods necessitate highly trained and skilled personnel, time-consuming detection process, complex pre-treatment steps, sophisticated and expensive instruments. However, biosensing and bio-analytics overcome these issues which are non-hazardous, and economical6.
Biosensors: The Concept
A biosensor is an analytical device which translates the modification of the physical or chemical properties of biological element or bio-matrix, due to the bio-electro-chemical interactions, into an electric signal whose amplitude is proportional to the concentration of the analyte in the solution7. The device consists of two basic components namely, a bio-recognition element, which is a detecting layer of immobilized elements like enzymes, antibodies, DNA, organelles, microorganisms etc., and a transducer of potentiometric, impediometric, amperometric, conductometric, acoustic, optic or colorimetric type7 as depicted in the figure 1. Amperometric biosensors are most widespread, numerous and successfully commercialized devices of bio-electronics and the research in this field is traceable from 1956 when Clark established his glucose electrode commonly called as Clark’s electrode. Amperometric biosensors measure the changes in the current on the working electrode due to direct oxidation of the analyte involving biochemical reaction or redox reaction8. However, conductometric transduction measures the changes in the conductance between a pair of metal electrodes as a consequence of the activity of an analyte. Enzyme reactions can be monitored by ion conductometric or impedimetric devices, using interdigitated microelectrodes9. The Potentiometric interpretation is based on the potential difference between an indicator and a reference electrode, or two reference electrodes separated by a selective membrane9. The advantage of amperometric detection is that significant enhancement in mass transport to the electrode surface. Selectivity due to the redox potential used for detection is characteristic of the analyte species1.
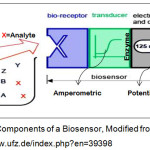 |
Figure 1: Components of a Biosensor, Modified from: https://www.ufz.de/index.php?en=39398 |
Laccase: As Bio Recognition Element
The enzyme laccase (polyphenol oxidase; EC 1.10.3.2) is a blue multi-copper-oxidase also categorized under oxidoreductase family. This enzyme has been choice of research for a long time, due to its versatility to oxidize a variety of phenolic and non-phenolic aromatic hydrocarbons. This enzyme has been extracted from a variety of biotic species like bacteria, fungi, plants, and insects mainly as extracellular enzymes1. Literature has reported that common sources of laccase are from Trametes versicolor, Aspergillus oryzae, Coriolus hirsutus, Cerrena unicolor, Pleurotus ostreatus, Rhus vernicifera, and also available commercially from these species in the markets. Laccases have an affinity for ortho- and para- substituted phenolic compounds but their affinity is usually higher towards the latter one. Laccases catalyze a wide variety of phenolic compounds, including mono-phenols, di-phenols, and polyphenols. Literature reports that laccase has been the model catalyst for substrates like aminophenols, methoxyphenols, aromatic amines, and ascorbate, with the concomitant four-electron reduction of oxygen to water10. This enzyme conjoins the four single-electron oxidations of the reducing substrate to the four-electron reductive cleavage of the di-oxygen bond with four copper atoms11. The copper atoms which forms the core of laccase are categorized into three groups depending on its characteristics obtained by UV/visible and electron paramagnetic resonance (EPR) spectroscopy. The type I copper atom (T1) is the reason for the intense blue color of the enzyme, which has strong electronic absorption of approximately 600 nm and is detectable by EPR. The type II copper atom (T2) is colorless but detectable by EPR and the pair of type III copper atom (T3) exhibits weak absorbance in the UV spectrum and no EPR response signal. The T2 and T3 copper atoms constitute a tri-nuclear cluster where the binding and multi-electron reduction of di-oxygen occur10, 11. The electrocatalytic mechanism of the laccase enzyme is initiated by the donation of an electron to the substrate by the T1 copper atom, followed by an inter electron transfer between reduced T1 to T2 and T3 copper sites. The role of T3 copper is to accept two-electrons in the aerobic oxidation process, for which the presence of the T2 copper is requisite. The reduction of oxygen to water takes place at the T2 and T3 cluster and passes through peroxide intermediate10. There are many advantages of laccase biosensors viz., laccase does not require H2O2 as co-substrate and any co-factors for its catalysis unlike tyrosinase and peroxidases, hence the construction of the biosensor is simple12, the applied potential will be within the optimum potential range established, influence to the response from analytes usually interfere in enzyme-based biosensors which is very small and the background current takes its smallest value in laccase biosensor13, and the molecular oxygen which is responsible for concomitant oxidation will be present in the carrier solution for the catalysis to happen13. The pictorial representation of the typical catalytic reaction of laccase is as shown in the figure 2.
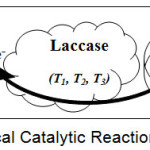 |
Figure 2: Typical Catalytic Reaction of Laccase. |
Considering immobilization of laccase, there are wide varieties of techniques employed till date by researchers. Most commonly used method is adsorption and apart from it, cross-linking, entrapment, encapsulation, covalent binding are also been reported. The benefit of an efficient protocol of laccase immobilization is very important for the prolonged use of the sensor and its anticipated extended storage and working stability1. The influencing factors on the immobilization methods are the type of transduction used (amperometric, potentiometric, impedimetric), the physic-chemical properties of the analyte (solubility, pH, temperature) and the operating conditions in which the biosensor is to function. All these considerations will allow the biological element (laccase) to exhibit maximum activity and help the stability and the reusability of the device14. Common methods of laccase immobilization are covalent binding, adsorption, cross-linking, encapsulation and entrapment. The functioning of three different laccase enzyme electrodes was studied based on immobilizing the laccase from Trametes versicolor on graphite electrodes by absorption and covalent binding which was studied in view of their use in agricultural or industrial waters polluted by phenolic compounds by Portaccio et al (2006). Further, the study concluded that electrochemical signals are being the characteristic of different immobilization methods studied and laccase immobilization using covalent bonds ensures higher sensitivities in comparison to adsorption15. Laccases are immobilized covalently with glutaraldehyde as the support matrix, which is the most common type of immobilization reported in the literature. However, adsorption is simple, low cost and fast technique where the enzyme is bound to a support via weak force such as van der Waals and hydrogen bridges. In encapsulation, the enzyme is confined to the core of suitable polymer16. The laccase enzyme retained its catalytic characteristics when entrapped in sol-gel silica as reported by Nur Atikah Mohidem and Hanapi Mat (2009). Entrapment uses a mixture of the biomaterial with a monomer solution which is then polymerized to a gel, to trap the enzyme within the interstitial spaces of the polymeric gel17. For example, Meiqing Guo et al (2014) developed amperometric catechol biosensor where the laccase was immobilized on nitrogen-doped ordered mesoporous carbon (N-OMC)/ polyvinyl alcohol (PVA). The laccase was entrapped in the matrix, regarded as novel biosensor with enhanced electrical activity during the catalysis of the substrate. Electro-polymerization has kindled focus among laccase immobilization strategies which is an electrochemical means to develop polymeric coatings by entrapping biomolecules which involve the application of appropriate potential on the working electrode immersed in the aqueous solution containing the electro-polymerizable monomer and enzyme18.
Sensitivity and Selectivity of Laccase Biosensors
The combination of Oxidoreductase enzyme and amperometric electrodes (transduction) is by far, the significantly studied enzymatic electrode concept, wherein various strategies of the enzyme reactions can be easily traced and sensitively measured by electrochemical means19. The laccase biosensor is of the third generation type where direct electron transfer occurs between the electrode and enzyme, hence their principle is attractive which requires no mediators and label-free. They are positively featured in high selectivity and sensitivity in the absence of interfering particles and interaction7. This section highlights the sensitivity and selectivity of the laccase enzyme sensor developed by many researchers for application in environmental analysis.
Suna Timur et al, (2004) developed a thick film electrode based biosensors containing Trametes versicolor (TvL), Aspergillus niger (AnL) laccases and Agaricus bisporus tissues (AbT) for the determination of phenolic compounds in wastewater. They used Polyaniline as a matrix for the immobilized laccase. The system was calibrated for different phenolic substances like phenol, catechol, L-DOPA with a maximum response time of 300 seconds and limit of detection in the range 0.2 to 20.0 µM for different analytes considered. Gautam et al, (2003) developed laccase biosensor on monolayer-modified with L-cysteine, cystamine, and 4-aminothiophenol on the gold electrode for quantification of catechol in range of 1 to 400 µM covalently coupled to the electrode surface. The developed sensor showed stability for 1 month without appreciable activity loss. Agnieszka et al, (2014) developed electrochemical laccase sensor to quantify hydroquinone and syringaldazine which are major toxicant found in groundwater, industrial effluents and surface water with a focus on the copolymer, N-alkylacridone derivative on a platinum electrode. The presented biosensor had a sensitivity of 2.34 ± 0.11 µAmM-1 and limit of detection of 0.93 µM for Hydroquinone. Priyanki et al, (2014) fabricated highly sensitive and stable laccase based amperometric biosensor on the nano-composite matrix, of osmium tetroxide on poly 4-vinylpyridine, multiwalled carbon nanotubes, Nafion and carbon black on glassy carbon working electrode for detecting pyrocatechol in environmental samples. The modified electrode worked in the linear range of 3.98 nM–16.71 nM with a minimum detection limit of 2.82 nM and a sensitivity of 3.82 ± 0.31 nA nM−1. Joanna et al, (2011) developed a hybrid phenol biosensor by electrolytic deposition of laccase from Cerrena unicolor on the surface of thin, ordered polythiophene films (copolymer) to determine the concentration of phenol, o-aminophenol, and catechol in the environmental samples. Research confirmed that the copolymer plays the essential role in the process of immobilization. Sarika et al (2014) designed and operated laccase based amperometric biosensor for industrial wastewaters to focus on covalent immobilization methods on a gold electrode. Laccase from Trametes versicolor was immobilized directly on gold electrode on one electrode (type A) by crosslinking with glutaraldehyde while, on the second electrode (type B), laccase was covalently bound to organothiol layers on gold electrode and finally on the third electrode (type C) laccase was covalently bound to silanized gold electrode with gold electrode of Clark type oxygen sensor. However, type B showed a highest correlation coefficient of 0.996 for quantification of catechol in the synthetic samples. The author here confirmed that it is possible to modulate the electrical response of laccase-based biosensors by using different immobilization methods directly on gold electrode of Clark type oxygen sensor. Anna et al (2005) studied on amperometric detection of mono- and diphenols from Cerrena unicolor laccase-modified graphite electrode by establishing correlation between sensitivity and substrate structure. The experimental data showed that among the ortho- or -para-substituted phenols, the sensitivity of the C. unicolor laccase-modified electrode increased in the following order -H, -CH3, -OH, -OCH3 and -NH3+ but in the case of para-substituted phenols, the Michaelis–Menten constants values were lower. The sensitivity indicated that the enzymatic oxidation products of the ortho-substituted phenols are more readily produced and re-reduced at the electrode with an increase in the amplification. Jegan et al (2005) used cross-linked enzyme crystals (CLEC) of laccase from Tramates Versicolor to develop biosensor for phenolic compounds which detected phenols in 50–1000 µ mol concentration level. The CLEC laccase retained appreciable activity for over 3 months and the optimum pH was 5.5-6.0 as reported in the study. Phenols with lower molecular weight such as 2-amino phenol, catechol, and pyrogallol gave a short response time ranging 120-140 seconds, whereas the higher molecular weight substrates like catechin and ABTS had comparatively a long response time up to 400 seconds. Jolanta et al (2008) constructed tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds like 2, 6-dimethoxyphenol, 4-tertbutyl catechol, 4-methylcatechol, 3-chlorophenol, and catechol. The highest sensitivity and the widest linear range was noticed for catechol but the author states that though bienzyme sensor is first of its kind but the stability of the proposed sensor was worst compared to other polyphenol biosensors. Advancement in the storability and usability of the laccase biosensor must be foreseen in the coming years of growing technological research.
Future Trend and Challenges
In this era of biological warfare omnipresent, the development of faster, reliable, accurate, robust, portable and low-cost biosensors gained utmost importance2. The sensitivity and selectivity of the existing laccase based biosensors are very high towards phenolic compounds and more improvements and advancements are anticipated in miniaturization of these devices and integration of the technological areas of surface chemistry, bioelectronics, and material chemistry2. Despite the past and current large amount of research in biosensor development, there is still a challenge to create improved, sophisticated and more reliable devices making use of molecular biology, nanotechnology, wireless communications systems, micro-fluidic devising, optical transduction, biochemistry, thin-film physics, and bio-electronics. However, as the world becomes more concerned about the impact that environmental contamination may cause on public health and the ecosystem, the demand for rapid detecting biosensors will increase22. The search for inexpensive supports and the recovery of activity during the immobilization process should be improved to increase the potential application of laccase immobilized systems5. Challenges are focused on, sensitivity of sensor in quantifying specks of environmental pollutants such as heavy metals, pathogens, toxins, and chemical toxicants in the environment; parallelism in terms of detecting multiple analytes; minimizing false positives; having rapid response without pretreatment of samples; transportability in terms of nanosized; easy to operate; affordability of sensors; and precision for the detection of single analyte. The global market shows about 10.4 % growth in the development of biosensors for various applications, like in biopharma, food processing and security, biodefense, and environmental analysis as reported by Vinod and Pratyoosh (2015).
Conclusions
In conclusion, phenolic compounds are micro polluting chemicals that are found in water environments and are known for their acute and chronic health effects on animals and humans. There is always a scope for a pragmatic approach towards quantification of the phenolic compounds and, biosensing is one of its kinds. The theme of biosensors must be confined to perform selective biological recognition of the target phenolic analyte in a complex sample matrix coupled with a sensitivity of electrochemical detection. It is imperative to research on screen-printed electrodes, nanostructure and nanomaterial engineering, optical transduction and microfluidic digitized electronics to achieve integration in bringing novel laccase biosensors. Developing a single biosensor with multi-analyte recognition capability has restricted its application. It is factual that portable electrochemical sensors for environmental applications are still in their infancy and are facing many challenges due to intrinsic characteristics of environmental analysis.
References
- Melissa M. Rodríguez-Delgado; Gibrán S. Alemán-Nava; José Manuel Rodríguez-Delgado; Graciano Dieck-Assad; Sergio Omar Martínez-Chapa; Damià Barceló; Roberto Parra. Laccase-based biosensors for detection of phenolic compounds. Trends Anal. Chem., 2015, 74, 21-45.
- Karim, F.; Fakhruddin, A. N. M. Recent advances in the development of biosensor for phenol: a review. Rev Environ Sci Biotechnol, 2012, 11, 261-274.
CrossRef - Maria-Pilar Marcoy; Damia Barcelo. Environmental applications of analytical biosensors. Meas. Sci. Technol., 1996, 7, 1547-1562.
CrossRef - Pratyoosh Shukla; Vinod Nigam; Rishi Gupta; Ajay Singh; Ramesh Chander Kuhad. Sustainable Enzyme Technology for Environment: Biosensors for Monitoring of Pollutants. Ramesh Chander Kuhad and Ajay Singh (eds.), Biotechnology for Environmental Management and Resource Recovery, Springer India, 2013, 69-76.
- Maria Fernández-Fernández; M. Ángeles Sanromán; Diego Moldes. Recent developments and applications of immobilized laccase. Biotechnol Adv., 2013, 31(8), 1808-1825.
CrossRef - Achintya, N. Bezbaruah; Harjyoti Kalita. Sensors and biosensors for endocrine disrupting chemicals: State-of-the-art and future trends. Jurate Virkutyte, S. Rajender Varma and Veeriah Jegatheesan (eds.), Treatment of Micropollutants in Water and Wastewater, IWA Publishing, London, UK, 2010, 93-127.
- Dzyadevych, S. V.; Arkhypova, V. N.; Soldatkin, A. P.; El’skaya, A. V.; Martelet, C.; Jaffrezic-Renault, N. IRBM, 2008, 29(2), 171-180.
CrossRef - Chawla, S.; Rawal, R.; Sharma, S.; Pundir, C. S. An amperometric biosensor based on laccase immobilized onto nickel nanoparticles/carboxylated multiwalled carbon nanotubes/polyaniline modified gold electrode for determination of phenolic content in fruit juices. Biochem. Eng. J., 2012, 68, 76-84.
CrossRef - Daniel Thevenot, R.; Toth, K.; Richard Durst, A.; Wilson, S. G. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron., 2001, 16, 121–131.
- Vernekar, M.; Lele, S. S. Laccase: Properties and Applications. BioResources, 2009, 4 (4), 1694-1717.
- Giardina. P; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: a never-ending story, Cell. Mol. Life Sci., 2010, 67, 369-385.
CrossRef - Roy, J. J.; Abrahama, T. E.; Abhijith, K. S.; Kumar V. P.S.; Thakur, M. S. Biosensor for the determination of phenols based on Cross-Linked Enzyme Crystals (CLEC) of laccase. Biosens. Bioelectron., 2005, 21, 206-211.
CrossRef - Anna Jarosz-Wilkołazka; Tautgirdas Ruzgas; Lo Gorton. Amperometric detection of mono- and diphenols at Cerrena unicolor laccase-modified graphite electrode: correlation between sensitivity and substrate structure. Talanta, 2005, 66, 1219–1224.
CrossRef - Singhm, M.; Verma, N.; Garg, A. K.; Redhu, N. Urea biosensors, Sensors and Actuators B, 2008, 134 (1), 345-351.
CrossRef - Portaccio, M.; Di Martino, S.; Maiuri, P.; Durante, D.; De Luca, P.; Lepore, M.; Bencivenga, U.; Rossi, S.; De Maioc, A.; Mita, D. G. Biosensors for phenolic compounds: The catechol as a substrate model. J. Mol. Catal. B: Enzym., 2006, 41, 97-102.
CrossRef - Rochefort, D.; Kouisni, L.; Gendron, K. Physical immobilization of laccase on an electrode by means of poly (ethyleneimine) microcapsules. J. Electroanal. Chem, 2008, 617(1), 53-63.
CrossRef - Pedro Ibarra-Escutia; Jorge Juarez Gómez; Carole Calas-Blanchard; Jean Louis Marty; María Teresa Ramírez-Silva. Amperometric biosensor based on a high resolution photopolymer deposited onto a screen-printed electrode for phenolic compounds monitoring in tea infusions. Talanta, 2010, 81 (4-5), 636- 1642.
CrossRef - Ozoner, S. K.; Erhan, E.; Yilmaz, F. Enzyme based phenol biosensors. Prof. Vernon Somerset (Ed.), Environmental Biosensors, InTech, 2011, 321-340.
- Jedrychowska, A.; Cabaj, J.; Swist, A.; Sołoducho, J. Electrochemical laccase sensor based on 3-methylthiophene/3-thiopheneacetic acid/bis(3,4-ethylenedioxythiophene)-Nnonylacridone as a new polymer support, J. Electroanal. Chem., 2014, 720-721, 64-70.
CrossRef - Sara Rodriguez-Mozaz; Maria-Pilar Marco; Maria J. Lopez de Alda; Damià Barceló. Biosensors for environmental applications: Future development trends, Pure Appl. Chem., 2004, 76 (4), 723-752.
CrossRef - Gupta, G.; Rajendran, V.; Atanassov, P. Laccase Biosensor on Monolayer-Modified Gold Electrode. Electroanalysis, 2003, 15 (20), 1577-1583.
CrossRef - Joanna Cabaj; Jadwiga Sołoducho; Antoni Chyla; Agnieszka Jedrychowska. Hybrid phenol biosensor based on modified phenoloxidase electrode. Sensors and Actuators B, 2011, 157 (1), 225-231.
CrossRef - Jolanta Kochana; Pawel Nowak; Anna Jarosz-Wilkołazka; Marta Bieron. Tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds. Microchem. J., 2008, 89 (2), 171-174.
CrossRef - Meiqing Guo; Hefeng Wang; Di Huang; Zhijun Han; Qiang Li; Xiaojun Wang; Jing Chen. Amperometric catechol biosensor based on laccase immobilized on nitrogen-doped ordered mesoporous carbon (N-OMC)/PVA matrix. Sci. Technol. Adv. Mater., 2014, 15, 1-9.
CrossRef - Das, P.; Barbora, L.; Das, M.; Goswami, P. Highly sensitive and stable laccase based amperometric biosensordeveloped on nano-composite matrix for detecting pyrocatechol inenvironmental samples. Sensors and Actuators B, 2014, 192, 737-744.
CrossRef - Sarika, C.; Rekha, K.; Narasimha Murthy, B. Laccase based amperometric biosensor for industrial wastewaters: A comparative study on covalent immobilization methods on gold electrode. IOSR Journal of Applied Chemistry, 2014, 7 (10), 20-27.
CrossRef - Suna Timur; Nurdan Pazarlioglu; Roberto Pilloton; Azmi Telefoncu. Thick film sensors based on laccases from different sources immobilized in polyaniline matrix. Sensors and Actuators B, 2004, 97 (1), 132-136.
CrossRef - Nigam, V. K.; Shukla, P. Enzyme Based Biosensors for Detection of Environmental Pollutants-A Review. J. Microbiol. Biotechnol., 2015, 25 (11), 1773-1781.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









