Comparative Study on Janus Kinase Enzyme Activity of Pomegranate Leaf Extract and its Active Component Ellagic Acid for Asthma
Sarithamol S, V. L. Pushpa and K. B. Manoj
PG and Research Department of Chemistry, Sree Narayana College, Kollam, Kerala, India, 691001
Corresponding Author E-mail: drpushpavl2017@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340255
Article Received on : October 13, 2017
Article Accepted on : January 10, 2018
Asthma is the most severe condition of lung airway inflammatory cells. Two third of asthmatic patients are nonresponsive towards existing treatments. The present study has focused on potency of IL4-JAK1-STAT6 signaling pathway as an investigational therapeutic target for the disease. Pomegranate plant is well studied for its potential phytochemicals. The therapeutic efficiency of pomegranate plant leaves and its active component ellagic acid against the signaling pathway has been investigated. The quantitative estimation of ellagic acid in the aqueous alcoholic extract of pomegranate leaves was done. Cell toxicity of ellagic acid and plant leaf extract was checked on macrophage raw cells using MTT assay and their activity against JAK1 enzyme were found out by ELISA method. The result signifies that pomegranate leaf extract is more efficient than ellagic acid towards JAK1 enzyme. Therefore the present study has highlighted the potency of pomegranate leaf extract over ellagic acid, the active component, for the disease asthma through IL4-JAK1-STAT6 pathway.
KEYWORDS:Asthma; JAK1-STAT6 Pathway; Phytochemical Extraction; HPLC; MTT Assay; Activity Assay
Download this article as:| Copy the following to cite this article: Sarithamol S, Pushpa V. L, Manoj K. B. Comparative Study on Janus Kinase Enzyme Activity of Pomegranate Leaf Extract and its Active Component Ellagic Acid for Asthma. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Sarithamol S, Pushpa V. L, Manoj K. B. Comparative Study on Janus Kinase Enzyme Activity of Pomegranate Leaf Extract and its Active Component Ellagic Acid for Asthma. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44301 |
Introduction
Asthma is a multi targeted anti-inflammatory lung disorder controlled by different type of pathways and proteins. Protein kinases are dominant class of targets for autoimmune diseases like asthma1. Inhibitors of protein kinases were established as effective drugs for asthma2. Corticosteroids, bronchodilators are some popular drugs in the market. Majority of asthma patients were nonresponsive towards those medications3. Cytokines are a category of potential targets for asthma. Their abnormal quantity during asthma attack made them to consider as potential targets4. Among the cytokines, interleukin 4, interleukin 5, interleukin 10, interleukin 13, interleukin 12 were identified as significant targets for the disease pathogenesis. After producing IL4 and IL13 by eosinophils and basophils, they get migrated to the inflammation site and produce mucus and immunoglobulin E, agents create problem while breathing. These cytokines communicate with their receptors through the cell surface and initiate the signaling process of janus kinase – signal transducer and activation of transcription pathways (JAK1-STAT6 pathways)5.
The present study has focused on IL4-IL4R signaling JAK1-STAT6 pathway. IL4 is an important biological target for the personalized treatment of asthma. janus kinase is one among the non receptor tyrosine kinase family is capable of initiating several signaling pathways including activation of signal transducer and activation of transcription (STAT) in mast cells, T cell, B cells, macrophages etc6. JAK family members include JAK1, JAK2, JAK3 and TYK2 and STAT family include STAT1 to 6 and STAT5A and 5B proteins. During the attack of allergens, cytokine communicate with its receptor, followed by the activation of JAKs. The activated JAKs phosphorylate specific tyrosine groups in the cytoplasmic region of cytokine receptor subunit. Those region then binds with STATs. The docking of STAT with tyrosine phosphorylated receptor subunit of IL4; they get phosphorylated by the activation of associated JAKs. The phosphorylated STAT then detached from the receptor subunit, dimerises and get migrated to the nucleus, there it cause gene transcription associated with asthma. Inhibiting the cytokine signaling pathway by suitable JAK inhibitors is highly recommended. The mechanism behind the inhibition is as follows. The active site of JAK is furnished by binding of an ATP (adenosine triphosphate) unit. One way of inhibition is that the inhibitor can behave as an ATP competitive inhibitor there by blocking the ATP binding to the catalytic cleft of JAK, followed by the deactivation JAKs7. The deactivated JAKs will never go for completion of the IL4 signaling pathway. Therefore JAK1-STAT6 signaling pathway inhibition in association with IL4-IL4R signaling can create an effectual result in the disease treatment. Nature is a rich source of compounds with high therapeutic potential8.
Plant world is an amazing example for it. Flavonoids, polyphenols and terpenes are some among the potential compounds. There are large variety of phytochemicals those having negative influence in the JAK1-STAT6 signaling pathway9. Anti-inflammatory phytochemicals such as flavonoids and curcuminoids are very good responsive towards JAK1. Present study focused on the therapeutic potential of one of the promising plants, pomegranate (Punicagranatum L., Punicaceae) against JAK1 enzyme10. Pomegranate plant is well appreciated for its antioxidant, anti-inflammatory anti cancerous properties. Its fruit, flower, bark, leaf etc. have independent therapeutic potential11. Here we are investigating the therapeutic effect of pomegranate leaf extract over its active principle, ellagic acid. The anti-inflammatory effect of aqueous alcoholic extract of leaves of pomegranate is due to the presence of ellagic acid as the major constituent. The present study focused on investigating whether ellagic acid or the extract is more active towards JAK 1 enzyme through invitro assay.
Materials and Methods
Materials
Ellagic acid (HPLC grade) purchased from Sigma Aldrich , JNK activity assay kit, Kinase star purchased from RayBiotech, Inc., RAW 264.7 cells was purchased from National Centre for Cell Sciences (NCCS), Pune, India and maintained Dulbecos modified Eagles medium ( Gibco, Invitrogen), MTT (Sigma, M-5655).
Extraction
Pomegranate leaves were collected locally and dried inthe shade at 40°C after washed well with running water. The dried material then ground into powder. The powder (10g) was macerated with 70% aqueous alcohol[70:30=alcohol: water] (100ml) at room temperature for seven days with occasional agitation. The extract was concentrated using rotary evaporator, dried under vacuum and stored at -80°C until use12.
HPLC Analysis of Extract
The extract was subjected to HPLC analysis for the quantification of ellagic acid in it. For the analysis, C-18 ODS2 HPLC column with diameter 5micrometer and dimension 4.6 150mm associated with a UV detector was orchestrated. The analysis was performed at a flow rate of 0.8ml/min. mobile phase consisted of acetonitrile (A) / O-phosphoric acid (0.3 %) in water (B). Absorbance was measured at a frequency of 254nm. Ellagic acid from the extract was estimated quantitatively using the pure ellagic acid as reference.
Invitro Analysis
The aqueous alcoholic extract and ellagic acid were subjected to invitro analysis with JAK 1 enzyme after checking their cytotoxicity towards RAW 264.7 macrophage cells using MTT assay. The procedure was as follows13
Different quantity of extract and ellagic acid (6.25,12.5,25,50,100 µg/mL) were incubated with seeded cells in 96 well plate at 1×104 cells/well in the culture medium at 37ºC in a humidified 5% CO2 incubator for 24 hours. After adding 15 mg of MTT, (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide), the system was subjected for 10 minutes incubation and the absorbance was measured using microplate reader at a wavelength of 540 nm. Results were produced by comparing with the percentage of untreated control. Any change in the morphology of the cells can be treated as cell toxicity.
Enzyme Activity Assay
LPS stimulated macrophage raw cells 264.7, were incubated with 100μl of leaf extract and ellagic acid at their maximum cell viable concentration for 37˚c overnight. After treatment with 200μl of blocking buffer (Composition – 0.2% gelatin in 0.05% tween20 in PBS), the system was incubated at room temperature for 1hour. 100µl primary antibody (JNK Specific antibody) was added and kept it for 2hours followed by treating with secondary antibody (100μl) for 1hr at room temperature. Finally 200µl of O-dianizdine (Composition – 1mg o – dianizdine/100ml methanol + 21ml citrate buffer+ 60ml hydrogen peroxide) was added and after 30minutes, stopped the reaction by adding 5N HCl (50μl).Read the absorbance at 415nm.
Result and Discussion
HPLC analysis
HPLC analysis on aqueous alcoholic extract of pomegranate leaves was carried out to quantify the ellagic acid in the extract. On examining the chromatogram in Fig 1, it was found that retention time corresponds to 10ppm of standard ellagic acid was 15.521 and that of X ppm of ellagic acid in plant extract was 15.421, the area under the peak of the standard sample was 922020 while that in the extract was 4857019. The amount of extract taken for HPLC analysis was 0.01g. The amount of ellagic acid in the extract can be quantified as follows
10ppm / X = 922020 / 4857019
X, quantity of the ellagic acid in the extract = (10x 4857019) / 922020
= (52.67802/ 0.01) x 10
= 52678.022ppm = 5.2678 %.
= 52.678 g/Kg
The quantity of ellagic acid in the pomegranate leaf aqueous alcoholic extract was found to be 5.267 %(52.678 g/ Kg). Ellagic acid is identified as the most significant therapeutic constituent of pomegranate plant. Through the present study, we are analyzing whether ellagic acid alone or extract as such is more significant for the JAK1 enzyme inhibition.
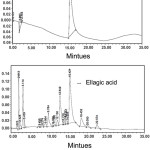 |
Figure 1: HPLC Chromatogram of Pure Ellagic Acid and Plant Extract. First Figure Represents Chromatogram of Ellagic Acid and Second Represents Plant Extract. Click here to View figure |
In vitro analysis on aqueous alcoholic extract of pomegranate leaves and ellagic acid
Invitro studies were conducted on the leaf extract and ellagic acid against JAK1 enzyme. Before that, the toxicity of extract and ellagic acid towards macrophage raw cells were checked. The toxicity studies of leaf extract and ellagic acid is explained as follows.
MTT assay for plant leaf extract (aqueous alcoholic extract) and ellagic acid
1mg of the dried leaf extract and ellagic acid were subjected to cytotoxicity studies on macrophage raw cells and the result is shown in the Fig 2 and Fig 3.
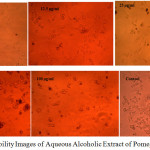 |
Figure 2: Cell Viability Images of Aqueous Alcoholic Extract of Pomegranate Leaf Click here to View figure |
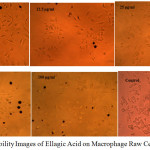 |
Figure 3: Cell Viability Images of Ellagic Acid on Macrophage Raw Cells |
The figures 2 & 3 gave clear information on the cyto toxicity of the leaf extract and ellagic acid. The maximum cell viability is observed only at lesser concentration of them. As the concentration increases, toxicity created by both the system increases. The cell viability is displayed in table 1. From the findings, the LD90 (concentration required for 90% viable cells) of extract was calculated as 0.1004µg and ellagic acid as 2.686 µg/ml (using the excel based statistical software LD50 plus). Fig 4 compares the toxic effect of leaf extract and ellagic acid
Table 1: Percentage Viability of Cells In Response With Varying Concentration Of Plant Extract And Ellagic Acid
| Concentration(µg/mL) | % viability Ellagic acid Leaf extract | |
| control | 100 | 100 |
| 6.25 | 73.86 | 75.3 |
| 12.5 | 68.15 | 60.36 |
| 25 | 68.23 | 56.99 |
| 50 | 57.62 | 56.82 |
| 100 | 49.92 | 46.12 |
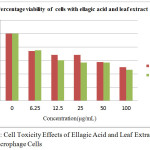 |
Figure 4: Cell Toxicity Effects of Ellagic Acid and Leaf Extract On Raw Macrophage Cells |
Enzyme Activity Assay
100μl of leaf extract (0.1004µg) and ellagic acid(2.686 µg) were subjected to activity assay with LPS induced macrophage raw cells and the percentage activity of both the samples were measured against JAK1 enzyme using JAK 1 specific antibody by indirect ELISA method. The percentage activity of extract towards JAK1 at 0.1004 µg concentration was found to be 66.53 % while that of ellagic acid at 2.686 µgconcentrations was found as 75.91 % (table 2). The result signifies potency of leaf extract over the active principle, ellagic acid.
Table 2: Concentration At 90% Cell Viable Condition of Macrophage Cells And Corresponding Activity Of Ellagic Acid And Leaf Extract
| Parameters | Ellagic acid | Leaf extract |
| Concentration(µg) | 2.97 | 0.1004 |
| Percentage activity | 75.91 | 66.53 |
Statistical significance of activity percentage data, student t test, p<0.005. Readings of data are average of three experiments.
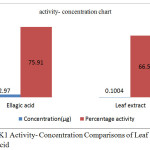 |
Figure 5: JAK1 Activity- Concentration Comparisons of Leaf Extract Vs Ellagic Acid |
The chart (Fig 5) shows that leaf extract is more efficient than the independent component, ellagic acid as JAK 1 inhibitor. The activity of leaf extract was found to be more than that of ellagic acid. The result is displayed in table 2. The findings signify the importance of extract as a better anti-inflammatory agent against JAK1. Therefore, the extract itself can be suggestively used for JAK1 driven immunomodulatory diseases like asthma, allergy, rheumatoid arthritis etc.
Conclusion
Pomegranate leaf extract and its active principle ellagic acid has compared for its activity against JAK1 enzyme which is a well-studied potential target for the disease asthma. A better inhibitor of JAK1 is able to act as a therapeutic agent for the disease. HPLC analysis quantifies the ellagic acid in the extract as 5.2678 % (52.678 g/Kg). MTT assay has revealed the cytotoxicity of leaf extract and ellagic acid against raw macrophage cells and both the systems were exhibiting better cell viability at lower concentrations. The percentage activity of both the samples against JAK1 enzyme was found to be 75. 91 % for ellagic acid and 66.53 % for leaf extract.The final result has highlighted the potency of leaf extract over the active principle ellagic acid as better JAK 1 inhibitor. This signifies the effectiveness of combination of potential compounds over individual ones. This inhibitory property can be making use of for designing drugs for asthma.
Acknowledgement
The authors are deeply indebted to ‘The University of Kerala’ for providing research fellowship. We express our genuine gratitude to ‘Sree Narayana College, Kollam’, Kerala for providing facilities as a research centre.
Author contribution
The manuscript was prepared through the contribution of all the authors.
Conflict of interest
The authors declare that there is no conflict of interest associated with the work
Reference
- Adcock,I.M.;Caramori ,G.;Chung,K.F.;Lancet.2008,372,1073–1087.
CrossRef - Hernández-flórez,D.;Valor,L.;Reum Clin.2017,12,91–99.
CrossRef - Barnes,P.J.; J Allergy Clin Immunol [Internet]. 2015,136,531–545.
CrossRef - Szabo,E.;Kovacs,I.;Grune,T.;Haczku, A.; Virag,L.; Eur J Allergy Clin Immunol. 2011,66,811–814.
- Shuai,K.;Liu,B.; www.nature.com/reviews/immunol. 2003,3,900–911.
- Wong ,W.S.F.; Pang, K.; Biochimica et Biophysica Acta.2004,1697,53–69.
CrossRef - Hodge,J.A.;Kawabata,T.T.;Krishnaswami,S.;Clark,J.D.;Telliez,J.B.;Dowty,M.E.;Menon,S.; Lamba, M.;Zwillich,S.; Clin Exp Rheumatol. 2016,34,318–328.
- Tanaka,T.; Takahashi,R.;Nutrients. 2013,5,2128–2143.
CrossRef - Chen,S.;Curr Drug Targets [Internet].2011,12,288–301.
CrossRef - Mohammad,S.M.;Kashani,H.H.;J Med Plants Res.2012,6,5306–5310.
CrossRef - Yoshimura,M.;Watanabe,Y.; Kasai,K.; Yamakoshi,J.; Biosci Biotechnol Biochem. 2014,8451,2368–2373.
- Chanda,S.V.; Food Anal. Methods.2012,5,396–404.
CrossRef - Dinesh,M.D.; Athira,P.S.; Ajma,N.;Abhisha,N.C.;Carmel,A.;Int J Life Sci Sci Res. 2016,2,412–414.

This work is licensed under a Creative Commons Attribution 4.0 International License.









