Coconut (Cocos nucifera L.) Lipids: Extraction and Characterization
1Department of Chemistry, Faculty of Science and Mathematics, Diponegoro University - Jl. Prof. Soedarto, SH 50 275 Semarang, Indonesia.
2Chemistry Program, Faculty of Science and Mathematics, Diponegoro University - Jl. Prof. Soedarto, SH 50 275 Semarang, Indonesia.
Corresponding Author E-mail: dwi.hudiyanti@live.undip.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340268
Article Received on : January 12, 2018
Article Accepted on : March 25, 2018
Lipids extraction from dried coconut meat is conducted by maceration using chloroform/methanol (2:1,v/v) and partition extraction with n-hexane/87% ethanol (3:1), and column chromatography. Lipids are analyzed using FTIR, GC-MS, and TLC. Results reveal that dryed coconut meat has 0.04% phospolipids, 0.22% of neutral lipids, and 0.04% of glycolipids and ceramides. Coconut phospolipids consist of phosphatidylcholine (PC), phosphatidylserine (PS), and/or phosphatidylethanolamine (PE) classes for the hydrophilic parts with dodecanoic acid (C12: 0), hexadecanoic acid (C16:0) and octadecanoic acid (C18: 0) as the lipophilic parts.
KEYWORDS:coconut; phospholipids; dodecanoic acid; hexadecanoic acid; octadecanoic acid
Download this article as:| Copy the following to cite this article: Hudiyanti D, Al-Khafiz M. F. Anam K. Coconut (Cocos nucifera L.) Lipids: Extraction and Characterization. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Hudiyanti D, Al-Khafiz M. F. Anam K. Coconut (Cocos nucifera L.) Lipids: Extraction and Characterization. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44959 |
Introduction
Phospholipids (PLs) are amphiphilic lipids obtained in cell membranes of all organisms, organized as lipid bilayers. The PLs discovered are mainly glycerophospholipids (GPLs), which are made of fatty acid residues (FAs) attached to a glycerol backbone by ester formation, a phosphate group and a hydrophilic head group (e.g. choline, serine or ethanolamine). Widespread supplies of industrially cultivated PLs are eggs (chicken and fish), bovine milk, soya, rapeseed, sunflower, coconut, sesame (1) and jack bean (2). Each source has a unique composition of distinct phospholipid species and accordingly diverse applications in pharmaceuticals (3), cosmetics (4), nutrition, food (5), and drug delivery (6,7).
In aqueous media, PLs molecules form aggregate structures by self-assembly course of action (8,9). Spherical bilayers known as vesicles or liposomes are among of these structures (10,11). Nowadays liposomes (7,12–14) and related structures (15,16) have been actively developed as carriers of bioactive compounds (13,14,17–19) in various applications (13,20) since their excellent biocompatibily to natural body environtment (6,12,21).
The characteristic of liposomes is greatly influenced by the physicochemical properties of PLs constituent, such as their molecular species (22–25). PLs molecular species are determined by their head group (hydrophilic) and hydrocarbon chain types (hydrophobic). These components are diversed base upon their sources (3). PLs acquired from various natural sources mostly have unique head groups and acyl chains compositions. A judicious stage in lipidomic study is lipid extraction with an suitable organic solvent combination (solvent system) preceding to MS analysis. The accomplishment of the lipid extraction by a certain solvent modification depends upon the partitioning of the lipids into the organic phase and therefore on the lipid composition (2,26,27).
Previously Hudiyanti et al. (25) obtained that crude coconut PLs contain sephaline groups whilst the lipophilic parts comprise of dodecanoic acid (C12:0) and octanoic acid (C8:0). In this study we take further steps by purifying these crude PLs with column vacuum chromatography using several eluents to learn more information about the chemistry of coconut PLs. Our new finding is that crude coconut PLs also contained phosphatidylcholine groups and the lipophilic components were hexadecanoic acid (C16:0) and 9-octadecenoic acid (C18:1) as well. Further details of this new finding is presented in the following discussions.
Materials and Methods
Materials
Dried coconut meat, filter paper, silica gel G60 for column chromatography, chloroform, acetone, methanol, aquabidest, NaCl, the solvent for partition was prepared by partitioning ethanol 87% with n-hexane (1: 1, v/v) and the top layer was mark solvent A while the bottom layer was solvent B, TLC plat of silica gel GF254 was washed with chloroform/methanol (1:1 v/v), then impregnate with 2.3% boric acid in absolute ethanol prior used. Spot regents for TLC were dragendorff, ninhidrin, and primulin reagents.
Extraction
Polar lipids from coconut meat was isolated by method previously presented by Hudiyanti et al. (25). Brieftly, this was prepared by the following procedure. Dried coconut meat powder was macerated with chloroform / methanol (2: 1) for 7 days. The filtrate was washed with a 0.9% of NaCl solution. Chloroform layer was evaporated to acquired coconut lipid extract. After that 10 g of coconut lipid extract was dissolved in 45 ml of solvent A and added to 15 ml solvent B in a separating funnel I. The mixture was shaken for 2 minutes. The bottom layer was transferred into a second separating funnel containing 45 ml solvent A and shaken for 2 minutes. The bottom layer containing polar lipids was collected in a vial for further purification while the upper layer was also kept in a separated vial. Fresh 15 ml solvent B was added to the remaining mixture in the first separating funnel and shaken for about 2 minutes. Then the bottom layer is transferred into the second separating funnel containing of fresh 45 ml solvent A. The mixture then shaken and separated as above. The bottom layer and the upper layer were accumulated into the previous separate containers. The procedure was repeated 4-6 times for each 10 g of coconut lipid extract. The polar lipids containing PLs were purified by column vaccum chromatography as described below.
Purification
A column containing 50 grams of silica gel G60 was prepared. A solution of the polar lipid/silica gel (1:1, w/w) was carefully placed on the silica gel column. For first elution we used chloroform to elute the remaining neutral lipids. Ten ml eluent was employed for each 10 mg of sample. Second eluent was acetone/methanol (9:1, v/v) to elute glycolipids and ceramides (with ratio: 5 ml eluent for 10 mg of sample) and followed by methanol to elute PLs. The coconut lipids was analyzed using FTIR, GCMS, and thin layer chromatography.
Results and Discussions
Extraction of lipids from dried coconut meat resulted in crude lipids extract, 49.03% of dried coconut meat. Furthermore the crude lipids extract contained 0.22% of neutral lipids, 0.04% of glycolipids and ceramides, and 0.04% of PLs.
The FTIR spectra of coconut crude lipids extract, neutral lipids, glycolipids and ceramides and PLs are presented in Figure 1. The major absorption peaks of lipids are evidently detectible in the spectrum. The C-H stretching vibrations can be identified by peaks at 2924 cm-1 and 2854 cm-1, C=O stretching of esters at 1744 to 1728 cm-1, CH2 bending at 1458 cm-1, CH3 symetric bending at 1373 cm-1, C-O-C stretching in esters at 1065-1250 cm-1, and CH2 rocking at 718 and 725 cm-1. Furthermore we find on the crude lipids extract and the PLs spectra several absorption peaks related to phosphate and choline groups. The phosphate group is recognized by PO2– asymmetric stretching peaks at 1226 cm-1 and 1219 cm-1, C-O-P stretching at 1111 cm-1 and 1064 cm-1 and P-O asymmetric stretching at 887 cm-1 and 817 cm-1. The choline group is recognised by (CH3)3N+ asymmetric bending at 1458 cm-1 and (CH3)3N+ asymmetric stretching at 972 cm-1 and 972 cm-1. Some of these peaks are slightly shifted from the characteristic band of lipids reported by Wolkers (28), Dean et al. (29) and Forfang et al. (30) it is assumed due to the interfering interaction between neighboring lipids such as acyl chain conformation and formation of hydrogen bonding.
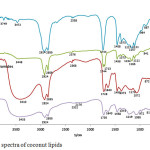 |
Figure 1: FTIR spectra of coconut lipids Click here to View figure |
The acyl chains (lipophilic part) of coconut crude lipid extract, neutral lipids, glycolipids and ceramides, and coconut PLs were analyzed by GCMS and presented on table 1. Data on table 1 shows that the composition of acyl chains found in coconut lipids are varied and range from C6 to C18:2. Crude lipids extract which is directly isolated from coconut meat by extraction with chloroform has the most varied acyl chain length and unsaturation compare to others. The variation decrease as the isolation proceed from crude lipids to PLs. Crude lipids extract has 9 types of acyl chains and 2 of them are unsaturated while the PLs has only 3 types and all of them are saturated i.e. C12:0, C16:0 and C18:0. The dodecanoic acid, C12:0, is the most abudant component in all of them. The dodecanoic acid composition in crude lipid extract, neutral lipids, glycolipids and ceramides, and coconut PLs are 45.85%, 48.13%, 68.15%, and 90.45% respectively. The data confirm that dodecanoic acid is the primary acyl chain in all type of lipids in coconut.
Table 1: Acyl chain components in coconut lipids
| Acyl chains |
Area (%) |
|||
| Crude lipids | Neutral lipids | Glycolipids &ceramides | PLs | |
| C6:0 (hexanoic acid) | 0,45 | – | – | – |
| C8:0 (octanoic acid) | 6,70 | 17,92 | 3,93 | – |
| C10:0 (decanoic acid) | 6,10 | 5,45 | 0,81 | – |
| C12:0 (dodecanoic acid) | 45,85 | 48,13 | 68,15 | 90,45 |
| C14:0 (tetradecanoic acid) | 19,61 | 2,13 | – | – |
| C16:0 (hexadecanoic acid) | 10,32 | 1,16 | 0,61 | 0,37 |
| C18:0 (octadecanoic acid) | 3,34 | 10,79 | 0,91 | 1,58 |
| C18:1 (9-Octadecenoic acid) | 6,34 | 2,01 | – | – |
| C18:2 (9,12-octadecadienoic acid) | 1,29 | – | – | – |
TLC analysis on coconut PLs using chloroform/methanol 9:1 as eluent we obtain four spots at Rf values 0.115; 0.385; 0.654; and 0.808 respectively, see figure 2. Dragendorff reagent reveals orange spots for Rf value 0.115 and Rf 0.808 which indicates the present of PLs containing choline groups (-CH2CH2N+(CH3)3), figure 3a. The spot at Rf value 0.385 turn to purplish red stain when ninhydrin reagent are used as spotting agent, figure 3b, which indicates the present of PLs containing free amino groups i.e. serine group (-CH2CH2N+H3(COOH)) or ethanolamine (-CH2CH2N+H3) groups. These results suggest that coconut PLs contain lipid from phosphatidylcholine (PC), phosphatidylserine (PS), and/or phosphatidylethanolamine (PE) classes.
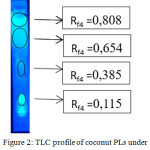 |
Figure 2: TLC profile of coconut PLs under UV lamp λ=365 nm |
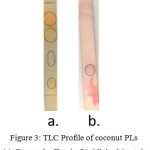 |
Figure 3: TLC Profile of coconut PLs (a). Dragendorff stain (b). Ninhydrin stain Click here to View figure |
Conclusions
Dryed coconut meat contain 0.04% phospolipids, 0.22% of neutral lipids, and 0.04% of glycolipids and ceramides. Coconut PLs comprise of phosphatidylcholine (PC), phosphatidylserine (PS), and/or phosphatidylethanolamine (PE) classes with dodecanoic acid (C12: 0), octadecanoic acid (C18: 0) and hexadecanoic acid (C16:0) as the lipophilic parts.
Acknowledgements
DH would like to ackowledge the financial support from The Minister of Research, Technology and Higher Education Indonesia via PUPT Research scheme 2016.
References
- Hudiyanti, D.; Raharjo, T.J.; Narsito, N.; Noegrohati, S. Orient J Chem. 2015, 31, 435–439.
CrossRef - Hudiyanti, D.; Arya, A.P.; Siahaan, P.; Suyati, L. Orient J Chem. 2015, 31, 2043–2046.
CrossRef - van Hoogevest, P.; Wendel, A. Eur J Lipid Sci Technol. 2014, 116, 1088–10107.
CrossRef - Djekic L.; Krajisnik D.; Micic Z. Tenside Surfactants Deterg. 2015, 52, 186–192.
CrossRef - Oosting, A.; Kegler, D.; Wopereis, H.J.; Teller, I.C.; van de Heijning, B.J.M.; Verkade, H.J.; van Der Beek, E.M. Pediatr Res. 2012, 72, 362–369.
CrossRef - Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. Asian J Pharm Sci. 2015, 10, 81–98.
CrossRef - Allen, T.M.; Cullis, P.R. Adv Drug Deliv Rev. 2013, 65, 36–48.
CrossRef - Marsh, D. Biophys J. 2012, 102, 1079–1087.
- Marsh, D. Biophys J. 2016, 110, 188–196.
CrossRef - Hudiyanti, D.; Raharjo, T.J.; Narsito, N.; Noegrohati, S. Indones J Chem. 2012, 12, 57–61.
CrossRef - Hudiyanti, D.; Radifar, M.; Raharjo, T.J.; Narsito, N.; Noegrohati, S. J Chem. 2014, 2014, e273084.
- Bozzuto, G., Molinari, A. Int J Nanomedicine. 2015, 10, 975–999.
CrossRef - Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Colloids Surf B Biointerfaces. 2009, 74, 114–122.
CrossRef - Drulis-Kawa, Z.; Dorotkiewicz-Jach, A. Int J Pharm. 2010, 1-2, 187–198.
CrossRef - Kato, S.; Aoshima, H.; Saitoh, Y.; Miwa, N.; J Photochem Photobiol B. 2010, 98, 99–105.
CrossRef - Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. J Colloid Sci Biotechnol. 2012, 1, 147–168.
CrossRef - Aisha, A.F.; Majid, A.M.S.A.; Ismail, Z. BMC Biotechnol. 2014, 14, 23.
CrossRef - Birchall, J.C.; Marichal, C.; Campbell, L.; Alwan, A.; Hadgraft, J.; Gumbleton, M. Int J Pharm. 2000, 197, 233–238.
CrossRef - Fočo, A.; Gašperlin, M.; Kristl, J. Int J Pharm. 2005, 291, 21–29.
CrossRef - Mo, R.; Jiang, T.; Gu, Z. Angew Chem Int Ed. 2014, 53, 5815–5820.
CrossRef - Mallick, S.; Choi, J.S. J Nanosci Nanotechnol. 2014, 14, 755–765.
CrossRef - Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Nanoscale Res Lett. 2013, 8, 102.
CrossRef - Kawasaki, M.; Yamashita, K.; Noda, M. In: 2016 IEEE SENSORS. 2016, 1–3.
- Saliba, A-E.; Vonkova, I.; Gavin, A-C. Nat Rev Mol Cell Biol. 2015, 16, 753–761.
CrossRef - Hudiyanti, D.; Raharjo, T.J.; Narsito, N.; Noegrohati, S. Agritech, 2012, 32, 23-26.
- Reis, A.; Rudnitskaya, A.; Blackburn, G.J.; Fauzi, N.M.; Pitt, A.R.; Spickett, C.M. J Lipid Res. 2013, 54, 1812–1824.
CrossRef - Axelsson, M.; Gentili, F. PLOS ONE. 2014, 9, e89643.
CrossRef - Wolkers, W.F.; In Biological and Biomedical Infrared Spectroscopy. 2009, 272–287.
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Bioresour Technol. 2010, 101, 4499–4507.
CrossRef - Forfang, K.; Zimmermann, B.; Kosa, G.; Kohler, A.; Shapaval, V. PLOS ONE. 2017, 12, e0170611.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










