Carbon–Carbon Bond Formation Reaction with Pd/reduced Graphene Oxide Composite
Padmakar Anant Kulkarni, Suresh Shamrao Shendage and Ashok Ganuji Awale
Department of Chemistry KET’S Vinayak Ganesh Vaze College of Arts, Science and Commerce Mithagar Road, Mulund (E) Mumbai-400081, India.
Corresponding Author E-mail: sureshsshendage@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340236
Article Received on : December 03, 2017
Article Accepted on : February 01, 2018
Palladium nanoparticles supported on reduced graphene oxide (Pd/rGO) catalyst was prepared by using alovera extract in sunlight. The catalyst (Pd/rGO) was employed for the Suzuki cross coupling reactions. The characterization of Pd/rGO was done by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray diffraction analysis (XRD). The average particle size of Pd was found to be 7±2 nm. The catalyst showed excellent catalytic activity with good recyclability for C- C cross coupling reactions.
KEYWORDS:Solar Energy; Alovera; Heterogeneous Catalysis; Greener Synthesis; Palladium Nanoparticles; Reduced Graphene Oxide; Cross Coupling Reactions
Download this article as:| Copy the following to cite this article: Kulkarni P. A, Shendage S. S, Awale A. G. Carbon–Carbon Bond Formation Reaction with Pd/reduced Graphene Oxide Composite. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Kulkarni P. A, Shendage S. S, Awale A. G. Carbon–Carbon Bond Formation Reaction with Pd/reduced Graphene Oxide Composite. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44181 |
Introduction
In last decades Suzuki–Miyaura cross-coupling reaction has gained considerable attention because of its use in making active pharmaceutical ingredients, agrochemicals, polymers, bioinorganic materials and natural products on a laboratory as well as industrial scale1. In 1981 the first method was reported for the preparation of biaryls via C-C cross-coupling reaction2. In general, homogeneous palladium complexes are used as catalysts in the Suzuki reaction3.
The reported homogeneous catalysts are found to have greater catalytic properties than heterogeneous catalysts in the formation of C-C coupling reactions4-6. The several researchers focused on the development of palladium complexes which efficiently catalyze the reaction7.
The Palladium complexes usually show greater catalytic activity. The main drawbacks of these catalysts are availability, stability, recovery, and high cost in addition to their sensitivity to air and moisture. So reactions catalyzed by Pd-complxes are carried under nitrogen atmosphere.
In order to overcome these challenges the efforts has been made to design heterogeneous Pd composites such as Pd supported on metal oxide, carbon, mesoporous zeolites and polymers, for use in Suzuki‐Miyaura coupling reactions8-10 Recently, the use of ligand- free heterogeneous palladium nanocomposites has drawn more attention of the researchers due to their simple handling, recovery, and recycling.
Further catalytic activity of Pd-based catalysts is increased by using different types of support materials11-14.The use of support facilitates stabilization and homogeneous dispersion of Pd supported catalyst15.
We have previously reported the electrochemical preparation of Pd NPs supported on graphene and their applications in suzuki coupling reactions11, 13 These catalysts exhibited high efficiency and easy recycling. The major challenges in palladium nanoparticle synthesis are size control and agglomeration of Pd NPs16, 17.
The synthesis of Pd NPs nanoparticles by greener route is one of the most striking features of present research18. In recent past various biological methods of synthesis of metal nanoparticles have been employed19-21. In biological methods metal nanoparticles are reduced by using bacteria, fungus and plant extracts as a reducing agents. The biological methods are more ecofriendly, avoid use of toxic reducing agents, and cheap. In literature few reports are available on synthesis of metal nanoparticles using sunlight 22-27. The main drawbacks these reported methods are high temperature operation, longer reaction time, toxic reducing agents and recovery of the catalyst. To overcome these drawbacks we herein, report synthesis of palladium nanoparicles supported on partially reduced graphene oxide (Pd/rGO) by using alovera extract and sunlight. In Aloe vera more than seventy five essential constituents such as enzymes, minerals, vitamins, sugars, lignin, saponins, amino acids and salicylic acids are present, which could facilitate the reduction of functional groups such as –COOH, -OH, epoxide, etc. in graphene oxide. The present protocol is novel, inexpensive and eco-friendly for the synthesis of Pd nanoparticles in which alovera acts as a mild reducing agent and sunlight as an energy source for the reduction. The catalytic application of prepared catalyst was examined in C-C Suzuki coupling reactions.
We report here the greener synthesis of Pd/rGO composite using alovera extract in sunlight and its applications in Suzuki coupling reactions. This environmentally benign catalyst is highly efficient, recyclable, stable for Suzuki coupling reactions.
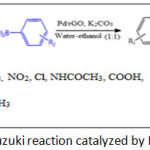 |
Scheme 1: Suzuki reaction catalyzed by Pd/rGO Click here to View scheme |
Experimenital
Material and Methods
All the reagents used were procured from Sigma-Aldrich of AR grade and used without any purification.
Synthesis of Graphene Oxide
The synthesis of Graphene oxide was carried out by reported modified methods in the literature 28. In typical procedure, 1 g of graphene and 1g NaNO3 was added to a round bottom flask containing 45 mL of concentrated H2SO4. The reaction mixture was maintained at 0-5°C for 2h and then charged 6g of KMnO4. The reaction mixture was diluted with water followed by heating at 98°C for15 min. A brown color solution was obtained after cooling reaction mixture to 30°C. The reaction mixture was then treated with 20 mL hydrogen peroxide till color change from brown to yellow. The reaction mass was quenched in 100 mL distilled water and stirred for 1h at 30°C. The solid obtained was centrifuged, washed with 10% HCl and finally with distilled water till neutral pH, and dried at 60°C.
Preparation of Aloevera Extract
100 g of fresh leaves of aloevera were chopped and mixed with 500 mL of distilled water. Then mixture was boiled and concentrated to 100 mL. This reaction mass was cooled and filtered to obtain aloevera extract. The prepared extract was stored in a refrigerator at 4°C
Synthesis of Pd/rGO Nanocomposite
A graphene oxide (800 mg) was charged to 500 mL beaker containing 200 mL distilled water. A PdCl2 solution (0.8 g) and 100 mL alovera extract was added to the reaction mixture and sonicated for 20 minutes at 28-30°C. The resulting mass was stirred under sunlight till the color changes from brown to black. The catalyst was filtered through whatman filter paper, washed with water, ethanol (50 mL), and dried at 110˚C to obtain a desired catalyst
Characterization of Pd/rGO Nano Composite
The morphological study of Pd/rGO was done by different analytical techniques such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM) and composition by X-ray Diffraction (XRD, Rigaku Miniflex model by using Cu Kα = 1.54 Å with scanning range 10–80°). Palladium content in the composite was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES).
General Procedure for Suzuki Coupling Reactions
A mixture of Aryl halide (1mmol), phenyl boronic acid (1.2 mmol), 3mmol potassium carbonate and 3 mg of Pd/rGO catalyst was charged in 5 mL ethanol-water (1:1) and refluxed for 6 h. The reaction was monitored by Gas Chromatography. After the completion, reaction mass was cooled the catalyst recovered by centrifugation. The recovered catalyst was washed with absolute alcohol, dried and used for next run. The pure products were obtained by column chromatography. The conversion of reactant was determined by Gas chromatography (GC). The final products of coupling reactions are characterized by 1HNMR and Mass spectroscopy.
Results and Discussion
(Pd/rGO) composite prepared by novel greener approach using alovera extract in presence of sunlight was characterized by ICP-AES, SEM, TEM and XRD.
The analysis of SEM image (Fig. 1) clearly shows the formation of Pd NPs on reduced graphene oxide with mono and uniform dispersion of Pd NPs.
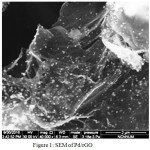 |
Figure 1: SEM of Pd/rGO |
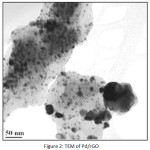 |
Figure 2: TEM of Pd/rGO |
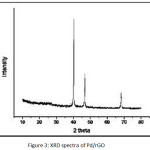 |
Figure 3: XRD spectra of Pd/rGO |
The TEM images (Fig. 2) show the presence of well dispersed Pd nanoparticles on rGO. The size of Pd NPs varies from 2-19 nm. Fig. 3 represents the XRD pattern of (Pd/rGO) composite. The broad weak diffraction peak appears at 24.61º, which indicates the reduction and exfoliation of GO into reduced graphene oxide. The peaks at around 2θ = 40.1º, 46.5º, 68.1º, for the Pd NPs on rGO are assigned to the (111), (200) and (220), crystalline planes of the face centered cubic structured palladium. It also confirms that the intensity of peak corresponds to 2θ = 40.1º is more and this could be major crystal facet. The average crystalline size was obtained by using Scherrer’s equation: D=0.9λ/βcosθ (where D is the average crystalline size, λ is the wavelength of Cu Kα, β is the fullwidth at half maximum of the diffraction peaks, and θ is the Bragg’s angle). The average crystalline size of PdNPs was found to be around 7±2 nm. The pd loading in Pd/rGO composite was found to be 7.2 wt.%.
In order to accomplish promising results, the effect of various solvents, bases, and catalyst amounts has been examined using iodobenzene and arylboronic acid as a representative reaction. Among the various solvents, ethanol-water mixture (1:1) was a better solvent (Table 1, entries 1-7). The reaction proceeded well in ethanol-H2O mixture compared to that in pure H2O or ethanol (Table 1, entries 8-9). Among the various bases K2CO3 (3 mol eq.) was identified as efficient base (Table 1, entries 1 and 10-13). The effect of catalyst loading was investigated. It is to be noted that the quantity of catalyst plays a significant role in the product yields. We assessed this reaction with various pd content from 0.1-0.3 mol%. The result demonstrates that 0.2 mol% palladium loading favours the reaction (Table 1, entries 14–17). The most favourable reaction conditions for the model reaction are Palladium: 0.2 mol%, Solvent: ethanol– water (50%, v/v), Base: K2CO3, and Temperature:t 80˚C.
Table1: Optimization of reaction parameters for Suzuki reaction catalyzed by Pd/rGOa
| Entry | Solvent | Base | Pd loading (mol %) | Time (h) | Yield (%)b |
| 1 | Ethanol/H2O | K2CO3 | 0.2 | 2 | 96 |
| 2 | Methanol | K2CO3 | 0.2 | 2 | 81 |
| 3 | Acetonitrile | K2CO3 | 0.2 | 2 | 53 |
| 4 | DMF | K2CO3 | 0.2 | 2 | 33 |
| 5 | Water/methanol | K2CO3 | 0.2 | 3 | 90 |
| 6 | DMF | K2CO3 | 0.2 | 4 | 22 |
| 7 | Water/toluene/1,2dioxane | K2CO3 | 0.2 | 6 | 22 |
| 8 | Water | K2CO3 | 0.2 | 12 | 20 |
| 9 | Ethanol | K2CO3 | 0.2 | 14 | 16 |
| 10 | Ethanol/H2O | Na2CO3 | 0.2 | 2 | 60 |
| 11 | Ethanol/H2O | TEA | 0.2 | 2 | 28 |
| 12 | Ethanol/H2O | N-methyl morpholine | 0.2 | 3 | 60 |
| 13 | Ethanol/H2O | NaOH) | 0.2 | 2 | 46 |
| 14 | Ethanol/H2O | K2CO3 | 0.3 | 2 | 96 |
| 15 | Ethanol/H2O | K2CO3 | 0.2 | 2 | 96 |
| 16 | Ethanol/H2O | K2CO3 | 0.15 | 6 | 61 |
| 17 | Ethanol/H2O | K2CO3 | 0.1 | 12 | 52 |
aReaction condition: Iodobenzene (1 mmol), phenyl boronic acid (1.2 mmol), base (3 mmol), solvent (5 mL), Catalyst Pd/rGO . Temp. 80˚C
bIsolated yield
The catalytic applicability of Pd/rGO was assessed for different substituents of aryl iodides and bromides (Table 2).
The aryl iodides with both electron donating and withdrawing substituents are found to be more reactive and gave exceptional yields within 2.0 h (Table 2, entries 2–10). The aryl bromides were less reactive in presence of the prepared catalyst. (Table 2, entries 12–15). The aryl iodide with bulky substituents also demonstrated good catalytic activity with moderate yields with a longer reaction time (Table 2, entry 11). The yields of reactions of 4-bromochlorobenzene, 4-iodoflurorobenzene, and 4-iodochlorobenzene with arylboronic acids were 94%, 89 %, 87% and 93% respectively (Table 2, entries 5, 12, 16 and17). The unsubstituted aryl iodide and bromide reacted efficiently with phenylboronic acid to give excellent yields (Table 2, entries 1, 6 and11) and less reactive cholorobenzene proceeded with longer reaction time.
 |
Table 2: Substrate study by Pd/rGOa |
aReaction condition: Ar-X (1 mmol), Ar-B(OH)2 (1.2 mmol), base (3.0 mmol), solvent (5 .0 mL), catalyst Pd/rGO (3.0 mg).Temp. 80°C
bIsolated yield
We have also investigated the recyclability of Pd/rGO catalyst for the aryl iodide with phenylboronic acid. The Pd/rGO catalyst was simply separated from the reaction mixture through centrifugation. Then it was washed with water and finally ethanol to remove the traces of products. It was then dried in air and used for the next runs. As seen from Table 3 prepared catalys tcould be successfully reused seven times without much loss in yield of the product. The loss in activity after 7th cycle could be due to agglomeration and increase in particle size of palladium nanoparticles on reduced graphene oxide.
Table 3: Recyclability studya
| Run | Ist | IInd | IIIrd | IVth | Vth | VIth | VIIth | VIIIth |
| Conversion | 100 | 100 | 99 | 98 | 96 | 95 | 93 | 89 |
aReaction condition: Iodobenzene (1 mmol), phenyl boronic (1.2mmol), base (3 mmol), solvent (5 mL), catalyst Pd/rGO (3mg). Temp. 80˚C
Conclusion
We have developed a novel ‘greener’ method for the preparation of Pd/rGO catalyst using alovera extract in sunlight. The various substituted aryl halides and substituted phenyl boronic acids were successfully coupled in the presence of prepared catalyst. This catalyst (Pd/rGO) showed high efficiency, stability and recyclability up to 7th cycles for the Suzuki–Miyaura C-C coupling reactions.
Acknowledgement
The authors are thankful to the KET’S V. G. Vaze College Mulund (E) Mumbai (INDIA) for funding.
References
- Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1439.
CrossRef - Miyaura, N.Yanagi T., Suzuki, A., Synth. Commun. 1981, 11, 513-519
CrossRef - Fan, H., Qi, Z.,Sui, D.,Mao, F.,Chen, R., Huang, J., Chinse J Catal. 2017, 38, 589-596
CrossRef - Dehury, N., Maity, N., Tripathy, S. K., Basset, J. M. , Patra, S.. ACS Catal 2016, 6, 5535-5540.
CrossRef - Nakayama, Y., Yokoyama, N., Nara, H., Kobayashi, T., Fujiwhara, M., Adv. Synth. Catal.2015, 357, 2322-2330
CrossRef - Heck, R. F, Acc. Chem. Res. 1979, 12, 146-151
CrossRef - Magano, J., Dunetz, J. R., Chem. Rev. 2011, 111, 2177-2250
CrossRef - Fu, W. Q., Feng, Y., Fang, Z. X., Chen, Q., Tang, T., Yu,Q. Y., Tang, T. D. Chem. Commun. 2016, 52, 3115-3118
CrossRef - Sun, R., Liu, B., Li, BG.,Jie, S. Y., Chem Cat Chem 2016, 8, 3261-3271
CrossRef - Kitamura,Y., Sako, S., Udzu, T., Tsutsui, A., Maegawa, T., Monguchi, Y., Sajiki, H.,Chem. Commun 2007, 5069-5071
CrossRef - Shendage, S. S., Patil, U. B., Nagarkar, J. M., Tetrahedron Lett 2013, 54, 3457-3461
CrossRef - Singh, A. S., Patil, U. B., Nagarkar, J. M., Catal Commun. 2013, 35, 11-16.
CrossRef - Shendage, S. S., Singh, A. S., Nagarkar, J. M., Tetrahedron Lett. 2014, 55, 857-860
CrossRef - Bedford, R. B., Singh, U. G., Walton, R. I., Williams, R. T., Davis, S. A., Chem Mater 2005, 17, 701-707
CrossRef - Ohtaka, A., Okagaki, T., Hamasaka, G., Uozumi, Y.,Shinagawa, T., Shimomura, O.,Nomura, R., Catalysts 2015, 5, 106-118
CrossRef - Nguyen, V. L., Nguyen, D. C, Hirata, H., Ohtaki, M., Hayakawa, T., Nogami, M. Adv. Nat. Sci: Nanosci. Nanotechnol 2010, 1, p35012
- Nemamcha, A., Rehspringer, J. L., Khatmi, D., J. Phys. Chem. B 2006, 110, 383-38
CrossRef - Narayanan, K. B., Sakthivel, N., Adv. Colloid Interface Sci . 2010 156, 1-13.
CrossRef - El-Hout, S. I., El-Sheikh, S. M., Hassan, F., Harraz, A., Ibrahim, I. A, El-Sharkawy, E. A. Appl. Catal. A 2015, 503, 176-185
CrossRef - Nasrollahzadeh, M., Mohammad, S., Sajadi Rostami-Vartooni, A., Alizadeh, M.,Bagherzadeh M., J Colloid Interface Sci 2016, 466, 360-368
CrossRef - Chettri, P., Vendamani, V. S., Tripathi, A., Singh, M. K., Pathak, A. P., Tiwari, A., Appl Surf Sci 2017, 406, 312-318.
CrossRef - Luo, Y., Mater Lett. 2008 62, 3770-3072
CrossRef - Luo, X., Colloid J 2009, 71, 281-284
- Chien, Y., Huang, C., Wang, S., Ye, C., Green Chem 2011 13, 1162-1166
CrossRef - Patil, A. B., Lanke, S. R., Deshmukh, K. M., Pandit, A. B., Bhanage, B. M., Mater Lett 2012, 79, 1-3
CrossRef - Patil, A. B., Bhanage, B.M., J Mol Catal A Chem. 2013, 379, 30-37
CrossRef - Patil, A. B., Bhanage, B. M., J Nanosci Nanotechnol 2013, 13, 5061-5068
CrossRef - Hummers, W. S., Offeman, R .E., J Am Chem Soc 1958, 80,1339
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









