An Investigation into Transesterification of Waste Cooking Oil
Hala Mohamed Abo-Dief1,2 , Ashraf Salah Emam3,4, Khamael Mohammed Abualnaja1 and Ashraf Talaat Mohamed5
, Ashraf Salah Emam3,4, Khamael Mohammed Abualnaja1 and Ashraf Talaat Mohamed5
1Chemical Dept., Faculty of Science, Al-Taif University, KSA.
2On leave from Egyptian Petroleum Research Institute Nasr City.
3Mechanical Eng. Dept., Faculty of Engineering, Albaha University, Albaha, KSA.
4On leave from Automotive and Tractors Dept., Faculty of Engineering, Mataria, Helwan University, Cairo, Egypt.
5Mechanical Engineering Department, Al-Baha Faculty of Engineering, Albaha University, KSA.
Corresponding Author E-mail: Mohamed.hala91@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340251
Article Received on : January 09, 2018
Article Accepted on : February 05, 2018
The present work is aiming at producing the biodiesel from corn waste frying oil and five fresh vegetable oils (corn, rapeseed, sunflower, soybean and palm). Three times used oil obtained. The transesterification process using KOH and NaOH at seven catalyst concentrations ranging from 0.2 to 1.2 %wt. Six reaction temperatures ranging from; 20 to 65 °C, test periods ranging from 0.5 h to 8.0 h and six alcohol/oil ratios have been used. Methanol was the alcohol of choice. The effect of test temperatures, test periods, alcohol/oil molar ratio and catalytic concentrations on both biodiesel %wt. and viscosity carried out and investigated. Non-used corn oil produces biodiesel with an efficiency reaching 98.5% followed by rapeseed, sunflower, soybean and palm respectively as compared to used oil that have 88% at catalytic concentration = 0.5, five hours, alcohol/oil molar ratio 5:1, impeller speed 500 rpm and 60oC. KOH is most effective than NaOH based catalysts.
KEYWORDS:Frying oil; Biodiesel; Vegetable oil; Used oil recycling
Download this article as:| Copy the following to cite this article: Abo-Dief H. M, Emam A. S, Abualnaja K. M, Mohamed A. T. An Investigation into Transesterification of Waste Cooking Oil. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Abo-Dief H. M, Emam A. S, Abualnaja K. M, Mohamed A. T. An Investigation into Transesterification of Waste Cooking Oil. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44819 |
Introduction
Energy is important for human existence and for socio-economic development. The development. The World’s economic growth affected by fuel price hike. Due to the expected depletion of crude oil encourage researches concerning the producing of renewable raw materials. There is a need to produce an energy source that is technically feasible, environmentally acceptable, economically competitive, and readily available, [1-2]. Abo-Dief et al. [3 and 4] produced oil from the used engine oil using the pyrolysis process and modified the produced oil characteristics, [5].
In all countries, diesel fuel is important in the industrial economy of all countries. It is a major part of the transport sector and their demand increasing steadily, [6]. Hossain and Boyce [7] and Demirbas [8] used biodiesel instead of petroleum-based diesel because it is a renewable, domestic resource with an environmentally friendly emission profile and is readily biodegradable. Santoria, Di-Nicolab, Moglieb, and Polonarab [9] discussed the technologies for producing biodiesel from vegetable seeds. Bouaid, Martinez, and Aracil [10] synthesed biodiesel from sunflower using KOH as catalyst. Leung, Wu, and Leung [11] reviewed the previous ways of reducing free fatty acids in raw oil and refinement of crude biodiesel. Dias, Ferraz, and Almeida [12] used KOH, NaOH and CH3ONa as catalysts for sunflower and soybean
frying oil. Sanli and Canakci [13] used methanol, ethanol, 2-propanol, and 1-butanol as alcohol in the transesterification of sunflower, corn, soybean, rapeseed, hazelnut, and cottonseed.
Anwar, Rashid, Ashraf, and Nadeem [14] and Sivasamy, Cheah, Fornasiero, Kemausuor, Zinoviev, and Miertus [15] showed that producing biodiesel from vegetables leading to alleviate food. Demirbas [8] defined that biodiesel composed of alkyl fatty acid with alcohols methanol or ethanol.
Kiakalaieh, Amin, and Mazaheri [16] used vegetable, waste and frying oils in producing biodiesel through transesterification process with alkaline catalysts. The nature of catalyst employed during transesterification reaction is crucial in converting triglycerides to biodiesel. The catalysts usually employed to catalyze transesterification reaction are homogeneous catalysts and heterogeneous catalysts. Thiruvengadaravi, Nandagopal, Baskaralingam, Bala, and Sivanesan [17] presented and discussed the quality of rapeseed-oil methyl esters (RME) used as biodiesel. Anwar, Rashid, Ashraf, and Nadeem, [14] derived alkali catalyst in producing biodiesel from okra seed. Hossain, and Boyce [18] used NaOH, KOH, CH3ONa, and CH3OK more often used in producing biodiesel. Endalew, Kiros, and Zanzi, [19] reviewed the development in using catalysts for the biodiesel production. Atadashi, Aroua, Abdul Aziz, and Sulaiman [1] analyzes the catalysts usage in the biodiesel production. Chesterfield, Rogers, Al-Zaini, and Adesina [20] used lipase-catalyzed ethanolysis of used cottonseed oil at temperatures between 297 and 348 K. The present work is aiming at producing the biodiesel weight% from corn waste frying oil and five fresh vegetable oils (corn, rapeseed, sunflower, soybean and palm). The effect of test temperatures, test periods, alcohol/oil molar ratio and catalytic concentrations on both biodiesel weight% and viscosity carried out and investigated.
Experimental
Five fresh types of oil; corn, sunflower, soybean, rapeseed and palm and three times frying corn oil are used with methanol and each of potassium and sodium hydroxide catalyst. Triplicate experiments carried for each set of operation variables using the transesterification method.
Seven concentrations of; 0.2, 0.4, 0.5, 0.6, 0.8, 1.0 and 1.2 wt.% of KOH and NaOH, six reaction temperatures of; 20, 30, 40, 50, 60 and 65 °C, test periods ranging from 0.5 h to 8.0 h and six molar alcohol/oil ratios of; 5, 6, 7, 9, 1, 11, and 13 used with methanol.
When each of the fresh vegetable and waste frying used oils added to the reactor individually with one of the catalyst that diluted in methanol, stirring started the reaction of the reactor additives for the predetermined time and temperature. As pressure and impeller speed remained constant, a fast conversion to ester (biodiesel) achieved after few minutes of the reaction starting. The mixture carefully transferred to a separating funnel and allowed to stand there overnight. After settling, the produced mixture separated into two liquid layers: crude ester layer on top and glycerol layer at the bottom. (The density of glycerol is much higher than that of ester, and it is insoluble in the ester phase). Lower layer (glycerol, methanol and most of the catalysts) drained out. The upper layer (methyl esters, some methanol and traces of the catalyst) cleaned thoroughly by washing with warm (50ºC) de-ionized water to remove soap, residual alcohol, catalyst, and unreacted tri-, di-, and mono-glycerides. The number of washing processes beyond four times does not have further benefit. The obtained ester conversion was calculated. The kinematic viscosity determined by a Cannon-Fenske viscometer tube (size no. 75) and Selecta VB-1423 viscosity bath. The methyl ester then dried with anhydrous Na2SO4.
Results and Discussions
Effect of Temperature on Biodiesel Weight%
Figures 1 and 2 illustrate the variation of biodiesel weight% with temperature using 0.5 concentration of KOH and NaOH catalyst at 6:1 Alcohol/oil weight ratio, and 60 min. It is clear that as the test temperature increases, the biodiesel weight% increasese for various oil types up to 60 oC. Further temperature increment leads encourage the saponification reaction of triglycerides that leads to the biodiesel weight% decrement inagreement with [11]. At lower temperatures, the amount of biodiesel is lower than that at higher temperatures because the process was incomplete inagreement with Refaat, Attia, Sibak, El Sheltawy, and ElDiwani [21]. The figure showed that the KOH catalyst trends of the amount of biodiesel wt. % shown higher compared with that of NaOH catalyst in agreement with [12]. Refaat, Attia, Sibak, El Sheltawy, and ElDiwani [21], Sanli, and Canakci [13] concluded that KOH is more superior to NaOH and that the KOH amount of 1.0% is appropriate. KOH concentration beyond 1.0% neither increases the product yield nor improves the obtained fuel’s properties. With NaOH usage, gel formed. The corn trend is shown higher compared with rapeseed, sunflower, soybean and palm respectively.
Effect of Alcohol on Biodiesel Weight%
Figures 3 and 4 illustrated the variation of biodiesel weight% with Alcohol/oil weight ratio concentration using KOH and NaOH catalyst concentration of 0.5 at 60oC, and 60 min. As, the alcohol/oil weight ratio increases, the biodiesel weight% increases up to 6:1, but beyond this ratio, the biodiesel weght% decreases in agreement with Leung, Wu, and Leung [11] due to both the conversion and the viscocity decrements. Also, Leung, Wu, and Leung [22] introduced that when the molar methanol ratio decreased from 6:1 to 3:1, the ester conversion dropped down, and both viscosity, density and total glycerol increased. Figure 3 showed that the trends of the amount of biodiesel wt.% in the case of using KOH catalyst shown higher compared with that of NaOH catalyst. The corn trend is shown higher compared with rapeseed, sunflower, soybean and palm respectively.
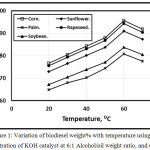 |
Figure 1: Variation of biodiesel weight% with temperature using 0.5 concentration of KOH catalyst at 6:1 Alcohol/oil weight ratio, and 60 min. |
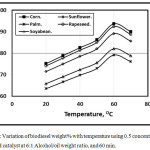 |
Figure 2: Variation of biodiesel weight% with temperature using 0.5 concentration of NaOH catalyst at 6:1 Alcohol/oil weight ratio, and 60 min. Click here to View figure |
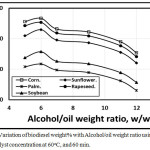 |
Figure 3: Variation of biodiesel weight% with Alcohol/oil weight ratio using 0.6 KOH catalyst concentration at 60 oC, and 60 min. Click here to View figure |
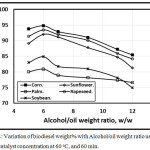 |
Figure 4: Variation of biodiesel weight% with Alcohol/oil weight ratio using 0.5 NaOH catalyst concentration at 60 oC, and 60 min. Click here to View figure |
Fig. 5 introduced the variation of biodiesel weight% with with Alcohol/oil weight ratio ranging from 2:1 to 14:1 using five different vegetable oils mentioned above and waste frying corn oil by transesterification with 1.0 NaOH catalyst concentration at 60oC, and 60 min. The optimum biodiesel wt. % found to be at alcohol: oil weight ratio at 6:1. The corn waste frying oil trend had the lower biodiesel weight % of 84%, while sunflower oil trend had the higher biodiesel wt. % of 98% in agreement with [13].
Effect of Catalytic Concentration on Biodiesel Weight %.
Figure 6 illustrated the variation of biodiesel weight% with KOH catalyst concentration at 5 Alcohol/oil ratio using at 60 oC, and 60 min. The biodiesel weight% increases with the catalystic concentration increases up to 0.6, with further catalystic concentration increment, the biodiesel yielding decreases due to the increment of soap formation in agreement with Leung, Wu, and Leung [11]. The biodiesel yielding in the case of used oil shown lower than that of the fresh oil for the same catalyst concentrations in agreement with Dias, Ferraz, and Almeida [12].
Effect of Test Period on % Biodiesel Weight.
Figure 7 illustrates the variation of biodiesel weight% with test period at 0.6 KOH catalyst concentration, 0.5 Alcohol/oil weight ratio using at 60 oC. As the test period increases, the biodiesel weight percentage increases for all types of virgin vegetable oil up to one hour, after one-hour test period, a rapid decrement of sunflower, rapeseed and palm occurred because the occurrence of fatty acid esters increases with reaction time in agreement with [13]. While there is an inflection after 6 hours test period for both corn oil and soybean oil. Sanli and Canakci [13] concluded that reaction durations longer than 1 h are not necessary.
Figure 8 showed the variation of viscocity with test period at 0.6 KOH catalyst concentration, 5 Alcohol/oil weight ratio using at 60 oC. It is clear that with increasing the test period, the viscosity decreases for various virgin oils due to the conversion of ester to oil in agreement with [13], they stated that after six hours test period, viscosity inflection occurs.
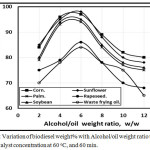 |
Figure 5: Variation of biodiesel weight% with Alcohol/oil weight ratio using 1.0 KOH catalyst concentration at 60 oC, and 60 min. Click here to View figure |
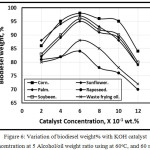 |
Figure 6. Variation of biodiesel weight% with KOH catalyst concentration at 5 Alcohol/oil weight ratio using at 60oC, and 60 min. |
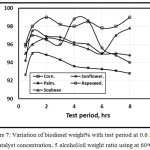 |
Figure 7: Variation of biodiesel weight% with test period at 0.6 KOH catalyst concentration, 5 alcohol/oil weight ratio using at 60oC. |
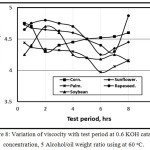 |
Figure 8: Variation of viscocity with test period at 0.6 KOH catalyst concentration, 5 Alcohol/oil weight ratio using at 60oC. |
Conclusions
The following conclusions obtained
Biodiesel production technologies based on vegetable crops are simple in operation and cost effective.
Fresh oil have higher biodiesel production efficiency of 98.5% using virgin oils for corn oil as compared to waste frying oils with a production efficiency of 88% at potassium hydroxide concentration = 0.5, five hours, Alcohol/Oil molar ratio 5:1, impeller speed 500 rpm and 60oC.
The optimum test condition are, alcohol/oil weight ratio = 6:1, 0.6 KOH catalyst concentration, 5 hrs test period and 60oC test temperature.
Fresh corn oil have higher biodiesel production efficiency of 98.5% followed by rapeseed, sunflower, soybean and palm fresh oils respectively.
At the optimum test conditions, as the test period increases, the viscosity decreases for all types of virgin oil except rapeseed.
References
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; and Sulaiman, N.M.; J. O. Ind. & Eng. Chem., 2010, 1-41.
- Kiakalaieh, A.T.; Amin, N.A.; and Mazaheri, H.; App. Energy, 2013, 104, 683-710.
CrossRef - Abo-Dief, H.M.; Altalhi, A.A.; and Mohamed, A.T.; J. O. Ind. and Intel. Inf. 2014, 2, 314-319.
- Abo-Dief, H.M.; and Mohamed, A.T.; The 29th annual meeting of the Saudi Biological Society, Dammam, Saudi Arabia on 2014, 426.
- Orabi, R.G.; Abo-Dief, H.M.; and Mohamed, A.T.; International Journal of Emergency Trends in Eng. & Develop. (IJETED), 2015, 3, 91-101.
- Abo-Dief, H.M.; Mostafa, N.A.; Alzahrani, E.S. and Mohamed, A.T.,”The Research J. Pharm Biol Chem Sci (RJPBCS), 2016, 4, 2999-3007.
- Hossain, A.B.; and Boyce, A.N.; Bulgarian Journal of Agricultural Science, 2009 4, 312-317.
- Demirbas, A.; Energy Conversion and Management, 2009, 50, 14–34.
CrossRef - Santoria, G.; Di Nicolab, G.; Moglieb, M.; and Polonarab, F.; Applied energy, 2012, 92, 109-132.
CrossRef - Bouaid, A.; Martinez, M.; and Aracil, J.; Chem. Eng. J., 2007, 134, 93–99.
CrossRef - Leung, D.Y.; Wu, X; and Leung, M.K.; J. App. Energy 2010, 87, 1083–1095.
CrossRef - Dias, J.M.; Ferraz, M.C.; and Almeida, M.F.; Fuel 2008, 87, 3572–3578.
CrossRef - Sanli, H.; and Canakci, M.; Energy & Fuels, 2008, 22, 2713–2719.
CrossRef - Anwar, F.; Rashid, U.; Ashraf, M.; and Nadeem, M.; Applied Energy, 2010, 87, 779–785.
CrossRef - Sivasamy, A.; Cheah, K.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; and Miertus, S.; Chem Sus Chem, 2009, 2, 278 – 300.
CrossRef - Kiakalaieh, A.T.; Amin, N.A.; and Mazaheri, H.N.; Applied Energy, 2013, 104, 683–710.
CrossRef - Thiruvengadaravi, K.; Nandagopal, J.; Baskaralingam, P.; Bala, V.; and Sivanesan, S.; Fuel, 2012, 98, 1–4.
CrossRef - Hossain, A.B.; and Boyce, A.N.; Bulgarian Journal of Agricultural Science, 2009, 15, 312-317.
- Endalew, A.; Kiros, Y.; and Zanzi, R.; Biomass and Bio Energy, 2011, 35, 3787- 3809.
CrossRef - Chesterfield, D.M.; Rogers, P.L.; Al-Zaini, E.O.; and Adesina, A.A.; Chem. Eng. J., 2012, 1-10.
- Refaat, A.A.; Attia, N.K.; Sibak, H.A.; El Sheltawy, S.T.; and ElDiwani, G.I.; Int. J. Environ. Sci. Tech., 2008, 5, 75-82.
CrossRef - Leung, D.; Wu, X.; and Leung, M.; Applied Energy, 2010, 87, 1083–1095.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









