An Efficient Synthesis and In Vitro Antimicrobial Screening of 2-Cyanoimino -4-aryl-6-(1,1'-biphenyl-4-yl)-3,4-dihydro-1H-Pyrimidines
Sivagami Swaminathan and Ingarsal Namasivayam
PG and Research Department of Chemistry, Rajah Serfoji Govt. College, Thanjavur-05. Tamil Nadu-India.
Corresponding Author E-mail: ningars@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/340222
Article Received on : January 01, 2018
Article Accepted on : February 05, 2018
An efficient synthesis of 2-Cyanoimino-4-aryl-6-(1,1’-biphenyl-4-yl)-3,4-dihydro-1H-pyrimidines from stryl-4-biphenylketones and cyanoguanidine in presence of sodium hydroxide has been described. Cyanoguanidine serves as N-C≡N source for the construction of desired cyanoiminopyrimidines. The structural assignments of the titled products were done accordingly to their spectra like Mass, FT-IR, Proton and Carbon-13 NMR spectroscopy. The more stable tautomeric form was ascertained using Computational frequency analysis. The tested microorganism profile of compounds exhibits significant antimicrobial activity.
KEYWORDS:Synthesis; 1-(1,1'-Biphenyl-4-yl)ethanone; Chalcone; Cyanoguanidine Cyanoiminopyrimidine; Microbial screening
Download this article as:| Copy the following to cite this article: Swaminathan S, Namasivayam I. An Efficient Synthesis and In Vitro Antimicrobial Screening of 2-Cyanoimino -4-aryl-6-(1,1'-biphenyl-4-yl)-3,4-dihydro-1H-Pyrimidines. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Swaminathan S, Namasivayam I. An Efficient Synthesis and In Vitro Antimicrobial Screening of 2-Cyanoimino -4-aryl-6-(1,1'-biphenyl-4-yl)-3,4-dihydro-1H-Pyrimidines. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44693 |
Introduction
Pyrimidines are very potent biological motifs among nitrogen containing heterocycles performs a unique function in cell metabolism and are the constituents of various types of alkaloids, antibiotics, vitamins etc1. They are exhibiting distinguished biological activities based on the functionalities present in their structures like antimicrobial2, antiplateletal3,anti-plasmodial4 etc. Aminopyrimidines are an important class of pyrimidine derivatives present in a number of naturally occurring compounds and are biologically active especially in DNA and RNA5. The properly substituted 2-aminopyrimidine derivatives have extensive applications in the medicinal field eg. drugs Meridianins (antitumor) 6, Diaveridine (antimicrobial)7, and Peroly-L-glutanin (anemia-treatment)8. Although large number of functionalities with different position in 2-aminopyrimidines were reported, the N-substitution in amino moiety possess a divergent pharmacological activities like Rosuvastatin (reduce cholesterol level) 9, tyrosine kinease inhibitor Gleevec10 etc.
The presence of flexible N-C-N connectivity in Cyanamides they are acting as useful intermediate, precursor etc. in the field of organic synthesis to produce pharmacologically important molecules. Cyanamides are interrelated to guanidines and are easily transformed or converted into one another11. Accordingly many cyanamide functionalized aromatics or heteroaromatics shows variety of biological activities such as anti-cathepsins K and L12, inhibition of spontaneous myogenic13, peptide activators14etc. The reported pharmacological importance of heterocyclic cyanamides emerged us to produce a simultaneous path way to synthesize these types of compounds and here by reporting the synthesis of cyanoimino pyrimidine with biphenyl moiety.
Materials and Methods
Chemicals used for the experiments were purchased from E-Merck and purified by standard procedures. Digital Melting point apparatus and silica coated aluminium TLC plates were used to confirm the melting points and purity of compounds in all stages. The instruments used for recording the spectra are Brucker AMX-400 MHz (NMR, Solvent CDCl3 and Tetra Methyl Silane reference), NICOLET AVATAR-360 (FT-IR, sample prepared with KBr as pellets) and CLASS-5000 (Mass) respectively.
Acetylation of Biphenyl (1)
4-Phenylacetophenone (2) was obtained by the addition of acetyl chloride to the reaction mixture containing dry AlCl3 and biphenyl in nitrobenzene with constant stirring15.
Synthesis of Styryl-4-biphenylketones (3a-g)
The round bottomed flask containing quantitative amounts of substituted benzaldehyde (20 mM) and 4-Phenylacetophenone (20 mM) in 60 ml of ethanol was heated to reflux and about 5 ml of sodium hydroxide solution (30 %) was added slowly during 15 minutes. The heating was continued till the completion of the reaction (nearly another 15 minutes). After cooling, the reaction mass was filtered and solid product was recrystalized using ethanol.
Synthesis of 2-Cyanoimino-4-aryl-6-(1,1′-biphenyl-4-yl)-3, 4-dihydro-1H-pyrimidines (4a-g). An ethanolic solution (60 mL) containing substituted Styryl-4-biphenyketone (3a-g) (10 mM), cyanoguanidine (10 mM) and aqueous sodium hydroxide (5 mL, 30 %) was refluxed for nearly three hours and the completion of reaction was confirmed using TLC. About 60 % of the solvent was recovered from the reaction mass under vacuum and slowly added to ice water and filtered the solid product. Column chromatography was used to isolate the pure products (mobile phase: ethyl acetate- benzene mixture).
Physical data and Spectral Assignments
2-Cyanoimino-6-(1,1′-biphenyl-4-yl)-4-phenyl-3,4-dihydro-1H-pyrimidine (4a).
M.F: C23H18N3, Yield % : 80, Melting Point°C : 98; IR ( cm-1): 3437 (N-H), 2185 (N ≡C), 1617 (N=C), 1515 ( C=C); 1H NMR (δ, ppm): 5.36 (4-H and 5-H, s), 6.37 (NH ,s), 7.24-7.62(NH and Aromatic,m) ; 13C NMR ( δ, ppm): 117.0 (N≡C) ,141.66 (6-C), 100.69 (5-C), 56.33(4-C), 155.87 (2-C) and 125.54-139.85 (Aromatic-C).Mass : Molecular ion (M+) peak m/z 350
2-Cyanoimino-6-(1,1′-biphenyl-4-yl)-4-(o-chlorophenyl)-3,4-dihydro-1H-pyrimidine (4b).
M.F: C23H17N3Cl, Yield % : 76 , Melting Point °C: 94; IR ( cm-1): 3209 (N-H), 2176 ( N ≡C ), 1624 ( N=C ), 1521 (C=C), 757 (C-Cl); 1H NMR (δ, ppm): 5.33 (4-H,d, J1,2= 4.2 Hz ), 5.82 ( 5-H,d, J1,2=4.2 Hz ), 6.42 ( NH, br.s), 7.25-7.70 (NH and Aromatic ,m); 13C NMR (δ, ppm): 116.99 (N≡C), 141.68 (6-C), 98.26 (5-C), 52.22 (4-C), 156.22 (2-C) and 125.62-140.48 (Aromatic-C).
2-Cyanoimino-6-(1,1′-biphenyl-4-yl)-4-( p-chlorophenyl)-3,4-dihydro-1H-pyrimidine (4c).
M.F: C23H17N3Cl, Yield % : 78, Melting Point °C: 125; IR (cm-1): 3187 (N-H), 2174 (N ≡C ), 1622 (N=C), 1519 (C=C)), 759 (C-Cl);1H NMR (δ, ppm): 5.25 (4-H,d, J1,2= 3.6 Hz), 5.36 (5-H,d, J1,2=3.6 Hz ), 6.54 (NH ,s ), 7.25-7.64 (NH and Aromatic ,m) ; 13C NMR (δ, ppm): 117.02 (N≡C) , 141.78 (6-C), 100.78 (5-C), 56.15 (4-C), 156.00 (2-C), and 125.60-139.91(Aromatic-C)
2-Cyanoimino-6-(1,1′-biphenyl-4-yl)-4-( p-methylphenyl)-3,4-dihydro-1H-pyrimidine (4d).
M.F: C24H20N3, Yield %: 70, Melting Point °C: 103; IR ( cm-1): 3205 (N-H), 2175 (N ≡C), 1620 (N=C), 1514 (C=C); 1H NMR (δ, ppm): 5.19 (4-H,s ), 5.27 (5-H, s), 6.45 (NH, s), 7.03-7.64 ( NH and Aromatic ,m), 2.16 (CH3 ,s); 13C NMR (δ, ppm): 21.21 (CH3),117.18 (N≡C) , 142.42 (6-C), 101.02 (5-C), 55.79 (4-C), 156.05 (2-C), and 126.22-139.97 (Aromatic-C).
2-Cyanoimino-6-(1,1′-biphenyl-4-yl)-4-( p-methoxyphenyl)-3,4-dihydro-1H-pyrimidine (4e).
M.F: C24H20N3O, Yield %: 69, Melting Point °C: 99; IR ( cm-1): 3314 (N-H), 2230 ( N ≡C ), 1657 ( N=C ), 1508 (C=C); 1H NMR ( δ, ppm): 5.21 (4-H, s ), 5.28 ( 5-H, s ), 6.29 (NH, s ), 7.22-7.52 (NH and Aromatic ,m), 3.81 ( OCH3, s ); 13C NMR ( δ, ppm): 55.57-55.40 (O-CH3) , 114.58 ( N≡C ), 142.52 (6-C), 101.01 (5-C), 56.29 (4-C) , 155.85 (2-C) and 125.57-139.92 (Aromatic-C)
2-Cyanoimino-6-(1,1′-biphenyl-4-yl)-4-( p -bromophenyl)-3,4-dihydro-1H-pyrimidine (4f).
M.F: C23H17N3Br, Yield %: 73, Melting Point °C: 72 ; IR ( cm-1): 3390 (N-H), 2183 ( N ≡C ), 1620 ( N=C ), 1510 (C=C), 754 (C-Br); 1H NMR (δ, ppm): 5.34 (4-H and 5-H, dis.d ), 6.93 ( NH, s ), 7.30-7.92 ( NH and Aromatic ,m); 13C NMR (δ, ppm): 115.02 (N≡C), 140.24 (6-C), 102.09 (5-C), 55.86 (4-C), 156.41 (2-C) and 123.12-134.56 (Aromatic-C)
2-Cyanoimino-6-(1,1′-biphenyl-4-yl)-4-( p-flurophenyl)-3,4-dihydro-1H-pyrimidine (4g).
M.F: C23H17N3F, Yield % : 67, Melting Point °C: 88; IR ( cm-1): 3450 (N-H), 2184 (N ≡C), 1659 (N=C), 1526 (C=C); 1H NMR (δ, ppm): 5.37 (4-H,d, J1,2=4.0 Hz), 5.77 (5-H, d, J1,2=4.0 Hz ), 6.59 ( NH, s ), 7.24-7.90 (NH and Aromatic ,m); 13C NMR (δ, ppm): 117.83 (N≡C), 144.61 (6-C), 99.42 (5-C), 53.27 (4-C), 157.03 (2-C) and 123.29-139.41 ( Aromatic-C).
Results and Discussion
Synthetic investigation of 2-Aminopyrimidines and their derivatives has received a considerable attention in the field of synthetic as well as medicinal chemistry. In this connection, cyanamides are well documented and are especially embedded in heterocyclic framework are an important structural unit of valuable synthetic intermediates for many medicinally relevant drugs such as diazepam, cyanoquanidine etc. Cyanamino pyrimidine contains N-cyanoguanidyl moiety in its structure and the cyanoguanidine moiety in organic cyanamides found in wide range of biological activities and chemical applications such as neuronal Na+ and Ca2+ channel blockers, glutamate release inhibitors, HIV-1 protease inhibitors, histamine H3 receptor antagonists etc16. Here we have constructed the cyanoimino pyrimidine moiety by using Cyano guanidine as the novel reagent via the following route:
Friedel-Crafts acylation of biphenyl (1) using acetyl chloride and dry AlCl3 gives 4-phenyl acetophenone (2). The reaction of (2) with substituted benzaldehydes in the presence of sodium hydroxide afford corresponding Styryl-4-biphenyketones (3a-g) and on further treatment with cyanoguanidine using sodium hydroxide in ethanol gives 2-Cyanoimino-4-aryl-6-(1,1′-biphenyl-4-yl)-3,4-dihydro-1H-pyrimidines (4a-g) (scheme-1).
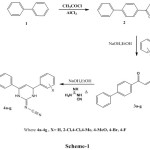 |
Scheme 1 Click here to View scheme |
The reaction of chalcone (3a-g) with cyanoguanidine in presence of base yields dihydro cyanoimino pyrimidine. The formation of this product might proceed either by route (i) 1, 4-addition or (ii) 1,2-addition of cyanoguanidine to the chalcone followed by the cyclisation of the intermediates ( scheme-2 ) like the mechanism proposed for 2-aminopyrimidines17.
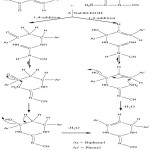 |
Scheme 2 Click here to View scheme |
The synthesized cyanamides (4a-g) are structurally conformed by analyzing their Mass, Infra red and Nuclear Magnetic Resonance spectral data. The IR spectral data’s are consistent with (stretching frequency, cm-1) NH (3180-3450), C=N (1610-1660), C=C (1515-1520) and C≡N(2175-2230) respectively for the synthesized cyanoiminopyrimidines . The proton NMR spectra of compounds 4a-4g reveals the presence of two D2O exchangeable NH protons in their structure, one in the region with chemical shift value around 6.3-6.9 ppm and another signal resonate in the aromatic region as merged signal. The benzylic (H-4) and ethylenic (H-5) protons present in the dihydropyrimidine moiety showed their respective signals at around 5.20-5.80 ppm.The protons H-4 and H-5 for compounds with electronegative substituent’s in the phenyl side chain like 4-fluoro, 2-chloro and 4-chloro showed two separate doublets with coupling constant J1,2=3.6-4.2 Hz. The compounds with phenyl and 4-bromophenyl rings give a single doublet for both H-4 and H-5 protons at around 5.35 ppm. But for the presence of electron rich methyl and methoxy groups as the substituent’s (4d and 4e) showed two separate singlets for protons at C-4 and C-5 position with same chemical shift region of other compounds.
The 13C NMR spectra of synthesized cyanoiminopyrimidines form an additional support for 1H and IR spectral assignments. The quaternary carbon between two nitrogen atoms at 2-C position resonates in the deshield region at155-157 ppm and the Nitrile carbon (C≡N) appears at 115-118 ppm. The remaining pyrimidine ring carbon signals appeared at 53-56 ppm (4-C), 98-102 ppm (5-C) and 140-144 ppm (6-C) respectively.
Accordingly the spectral evidence suggest the formation of 2-cyanoiminopyrimidine in the following tautomeric structures T-1, T-2 and T-3. From that the more stable tautomeric form is ascertained using quantum mechanical calculation, [DFT-B3LYP/6-31g(d)] method.In this method the molecular geometrics of the tautomers were optimized and the stationary points are characterized as minima by computation frequency analysis and thus to find the position of NH protons.
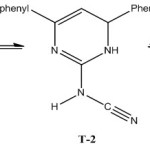 |
Scheme 3 |
The dipole moments, absolute and relative energies of possible tautomers T-1, T-2 and T-3 are displayed in Table-1.Among the tautomers, T-3 is more stable and T-1 is least stable.
Table 1: Calculated Thermodynamic parameters of T-1, T-2 and T-3
| Tautomer | µ (Debey) | E ( a. u) | ΔE kcal/mol |
| A | 7.03 | -1106.259089 | 18.29 |
| B | 2.15 | -1106.29737 | 11.60 |
| C | 8.51 | -1106.2882228 | 0.0 |
As a result of theoretical DFT calculation about the energies of tautomers indicates that the presence of NH protons as in the pyrimidine ring is more favored for cyanoiminopyrimidine and not for other tautomers T-1 and T-2. The possibilities of tautomers with more stable form of synthesized cyanoiminopyrimidines were reported in earlier findings as same 18, 19.
Antimicrobial Activity
Compounds 4a-4g were evaluated for their antimicrobial activity against two fungal and four bacterial strains. As shown in the table-2, for the phenyl ring attached to the 4th-position of the pyrimidine ring with bromo / fluro groups (4f and 4g), the best activities were obtained for both bacterial and fungal profiles. Between these two, the compound 4g exhibit more activity. Among the synthesized cyanoiminopyrimidines, except compounds 4a and 4b, all other shows moderate to better activity compared to the standard moxifloxacin and Amphotericin B. The antifungal activity of compounds 4a-4g showed the best results as compared to antibacterial activity. In addition, the compounds 4c and 4g are best active and comparable with standard against Escherichia Coli and Bacillus Subtilis.
Table 2: Antimicrobial Activity of compounds 4a-4g
| S. No. | Microorganism | Inhibition zone in mm | |||||||||
| Bacterial strains | DMSO(control) | Reference* | 4a | 4b | 4c | 4d | 4e | 4f | 4g | ||
| 1 | Escherichia coli | – | 28 | – | – | 28 | 19 | 20 | 20 | 21 | |
| 2 | Klebsiella pneumonia | – | 26 | – | – | – | 14 | 16 | 16 | 18 | |
| 3 | Bacillus subtilis | – | 28 | – | – | 18 | – | 24 | 26 | 22 | |
| 4 | Staphylococcus aureus | – | 32 | 12 | 15 | 16 | 16 | 18 | 20 | 22 | |
| Fungal strains | |||||||||||
| 5 | Aspergillus flavus | – | 08 | 07 | 07 | 08 | 09 | 10 | 10 | 10 | |
| 6 | Aspergillus niger | – | 12 | 07 | 07 | 10 | – | – | 12 | 12 | |
*Moxifloxacin (Bacteria) *Amphotericin B (Fungi)
Acknowledgements
The authors acknowledge Annamalai University (Annamalai Nagar), SASTRA University (Thanjavur) and Indian Institute of Technology, Chennai, Tamil Nadu-India for providing spectral facilities.
References
- Radi, M.; Schenone, S.; Botta, M. Org.Biomol.Chem., 2009, 7, 2841-2847
CrossRef - Suresh, L.; Poornachandra,Y.; Kanakaraju,S.; Ganesh Kumar,C.; Chandramouli,G.V.P.Org. Biomol. Chem., 2015, 13, 7294–7306.
CrossRef - Esfahanizadeh, M.; Mohebbi, S.; Bozorg, B.D.; Amidi, S.; Gudarzi,A.; Ayatollahi,S.A.; Kobarfard, F. Iran. J.Pharm.Research, 2015, 14 (2), 417-424
- Singh,K.; Kaur,H.; Chibale,K.; Balzarini,J.;Little,S.;Bharatam,P.V. Eur.J.Med.Chem., 2012,52, 82-97
CrossRef - Koroleva, E. V.; Gusak, K. N.; Ignatovich, Z. V. Russ. Chem. Rev., 2010, 79(8), 655-681
CrossRef - Walker, S. R.; Carter, E. J. ; Huff, B. C.; Morris, J. C.Chem. Rev., 2009, 109(7), 3080-3098
CrossRef - Brossi, A.; Hoffer, M.; Grunberg, E.; Mitrovic, M. J. Med. Chem., 1971, 14(5), 462-463
CrossRef - Balaban, A. T.; Oniciu, D. C.; Katritzky, A. Chem. Rev., 2004, 104(5), 2777-2812
CrossRef - Andrushko. N.; Andrushko, V.; Konig, G.; Spannenberg, A.; Berner, A. Eur. J. Org. Chem., 2008, 5, 847-853
CrossRef - Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Nat.Rev. Drug Discovery, 2002, 1, 493-502
CrossRef - Larraufie, M.H.; Ollivier, C.; Fensterbank, L.; Malacria, M.; Lacote, E. Angew. Chem. Int. Ed., 2010, 49, 2178 –2181
CrossRef - Barrett, D. G.; Deaton, D. N.; Hassell, A.M.; McFadyen, R. B.; Miller, A. B. ; Miller, L. R.; Payne, J. A.; Shewchuk, L. M.; Willard, D. H.Jr.; Wright, L. L. Bioorg. Med. Chem. Lett., 2005, 15, 3039–3043;
CrossRef - Manley, P. W.; Quast, U. J. Med.Chem., 1992, 35, 2327–2340
CrossRef - Danger, G.; Michaut, A.; Bucchi, M.; Boiteau, L.; Canal, J.; Plasson, R.; Pascal, R. Angew. Chem., Int. Ed., 2013, 52, 611–614.
CrossRef - A.I. Vogel. Elementary Practical Organic Chemistry, Part 1: Small scale preparations, ELBS Publishers, New York, 1966.
- Katla,V.R.; Syed,R.; Kuruva,C.S.; Kuntrapakam,H.K.; Chamarthi,N.R. Chem.Pharm.Bull., 2013, 61(1),25-32
CrossRef - EL-Rayyes, N. R. J. Heterocycl. Chem., 1982, 19(2), 415-419
CrossRef - Moustafa, A. H.; Shestakov, A .S .; Shikhaliev, K. S. Chem. Heterocycl. Compd., 2012, 48(4), 613-619.
CrossRef - Hulme, R.; Zamora, O. D. P.; Mota, E. J.; Pasten, M. A.; Contreas-Rojas,R.; Miranda, R.; Valencia-Hernandez, I.; Correa-Basurto, J.; Trujillo-Ferrara, J.; Delgado, F. Tetrahedron, 2008, 64(15), 3372-3380.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









