A Decade of Development of Ethylidenethiosemicarbazides as Building Blocks for Synthesis of Azoles and Azines (A Review)
Sayed M. Riyadh1,2 , Shojaa Abed El-Motairi1 and Anwar A. Deawaly1
, Shojaa Abed El-Motairi1 and Anwar A. Deawaly1
1Department of Chemistry, Faculty of Science, Taibah University, Almadinah Almunawrah, 30002, Saudi Arabia.
2Department of Chemistry, Faculty of Science, Cairo University, Giza, 12613, Egypt.
DOI : http://dx.doi.org/10.13005/ojc/340201
Article Received on : February 26, 2018
Article Accepted on : March 30, 2018
This survey represents synthesis of ethylidenethiosemicarbazides, with aryl or heterocyclic moieties, and its utility as building blocks for construction of different heterocyclic compounds such as; thiazoles, thiazolidinones, [1,3,4]thiadiazoles, [1,3,4]triazoles, [1,3]thiazines, pyrimidines, thiazolo[5,4-b]quinoxalines, bis-thiazoles, and bis-pyrazoles. Also, the pharmaceutical applications of ethylidenethiosemicarbazides have been demonstrated.
KEYWORDS:Thiosemicarbazones; Azoles; Azines; Cyclocondensation; Biological Activity
Download this article as:| Copy the following to cite this article: Riyadh S. M, El-Motairi S. A, Deawaly A. A. A Decade of Development of Ethylidenethiosemicarbazides as Building Blocks for Synthesis of Azoles and Azines (A Review). Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Riyadh S. M, El-Motairi S. A, Deawaly A. A. A Decade of Development of Ethylidenethiosemicarbazides as Building Blocks for Synthesis of Azoles and Azines (A Review). Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44998 |
Introduction
Ethylidenethiosemicarbazides [(ethylidene)hydrazine carbothioamides) are a class of organic compounds with general structure [Ar(CH3)C=N-NH-CS-NH2]. Variation of substituents on thioamide nitrogen (A), sulfur atom (B), and hydrazone nitrogen (C) led to developing an array of ethylidenethiosemicarbazides with structural diversity and broad spectrum of biological activities (Fig. 1).
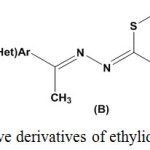 |
Figure 1: Representative derivatives of ethylidenethiosemicarbazides
|
Recently, ethylidenethiosemicarbazides have been used as effective pharmacophoric agents in medicinal chemistry such as; antitumor1, anti-tubercular2, anti-amoebic3, anti-fungal4, antiviral5, antimicrobial6, antioxidant7, anticonvulsant8, and anti-trypanosomal9,10. Also, these compounds were prescribed for treatment of hypertension as calcium channel blockers11. The biological activity of ethylidenethiosemicarbazides is related to their chelating ability with metal ion through either thione or thiolate sulfur and one of the hydrazone-nitrogen atoms12. Furthermore, ethylidenethiosemicarbazides have been used as a reactive building blocks for synthesis of different azoles such as, bis-thiazoles13, [1,3,4]thiadiazoles14, [1,3,4]oxadiazoles15, thiazolidin-4-ones16, imidazolinones17, and thiazoles18,19. In this survey, we represent three approaches about ethylidenethiosemicarbazides, with different aryl or heterocyclic moieties, including synthesis, reactivity, and their biological activities.
Synthesis of Ethylidenethiosemicarbazides
Ethylidenethiosemicarbazides with aryl group (compound 3) or heterocyclic moiety (compound 5) were prepared through condensation reactions of thiosemicarbazide (1) with acetylarene 2 or heterocyclic ethanone 4, respectively [Scheme 1 & Tables 1,2].
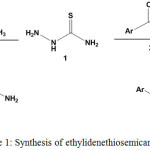 |
Scheme 1: Synthesis of ethylidenethiosemicarbazides |
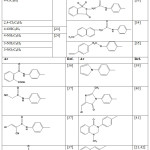 |
Table 1: 1-(1-arylethylidene)thiosemicarbazide derivatives |
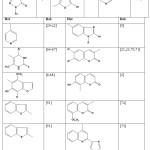 |
Table 2: Ethylidenethiosemicarbazides with heterocyclic moiety |
Reactions of Ethylidenethiosemicarbazides
Reaction with a-Ketohydrazonoyl Halides
5-Arylazothiazoles 7 were synthesized via reactions of ethylidenehydrazine-1-carbothioamides 3 or 5 with a-ketohydrazonoyl halides 6 [N-aryl 2-oxopropanehydrazonoyl chlorides or N-aryl 2-substituted-acetohydrazonoyl bromides] under thermal conditions6,7,18,19,42,43,53,57,70,80-86 (Scheme 2).
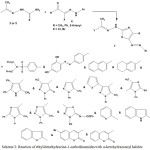 |
Scheme 2: Reaction of ethylidenehydrazine-1-carbothioamides with a-ketohydrazonoyl halides |
By analogous method, ethylidenehydrazine-1-carbothioamides 3 or 5 reacted with ethyl N-aryl-2-chloro-2-hydrazono acetate 8 under reflux conditions in dioxane, containing catalytic amount of triethyl amine and afforded arylhydrazothiazolone81,85 derivatives 9 (Scheme 3).
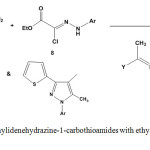 |
Scheme 3: Reaction of ethylidenehydrazine-1-carbothioamides with ethyl N-aryl-2-chloro-2-hydrazono acetate |
The reactivity of thiosemicarbazones 3 or 5 towards N-aryl carbohydrazonoyl chlorides 10, without keto group, has been reported18,19,84 under thermal19,84 or microwave irradiation and using grafted chitosan18 or triethylamine19,84 as basic catalyst. These reactions have been established to give [1,3,4]thiadiazoles 11 (Scheme 4).
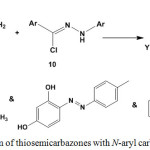 |
Scheme 4: Reaction of thiosemicarbazones with N-aryl carbohydrazonoyl chlorides |
Reaction with bis-hydrazonoyl Chlorides
Two equivalents of thiosemicarbazones 3 or 5 were reacted with bis-[a-ketohydrazonoyl chlorides] 12 in dioxane, in the presence of catalytic amount of triethylamine, to furnish the corresponding bis-(hydrazonothiazoles)87 13 (Scheme 5).
![Scheme 5: Reaction of thiosemicarbazones with bis-[a-ketohydrazonoyl chlorides]](http://www.orientjchem.org/wp-content/uploads/2018/04/Vol34No2_Dec_Say_sch5-150x150.jpg) |
Scheme 5: Reaction of thiosemicarbazones with bis-[a-ketohydrazonoyl chlorides] |
By the same manner, bis-hydrazonothiazoles 15, containing sulfonyl group, were synthesized via reaction of thiosemicarbazones 3 or 5 with sulfonyl bis-[a-ketohydrazonoyl chlorides] 14 in a molar ratio (2:1), respectively87 (Scheme 6).
![Scheme 6: Reaction of thiosemicarbazones with sulfonyl bis-[a-ketohydrazonoyl chlorides]](http://www.orientjchem.org/wp-content/uploads/2018/04/Vol34No2_Dec_Say_sch6-150x150.jpg) |
Scheme 6: Reaction of thiosemicarbazones with sulfonyl bis-[a-ketohydrazonoyl chlorides] |
Reaction with a-halocarbonyl Compounds
Treatment of 2-[(1-arylethylidene)hydrazine]-1-carbothioamides 3 with 1-aryl-2-bromoethanone 16 under thermal conditions gave the respective 2-[2-(1-arylethylidene)hydrazono]-4-arylthiazoles6,10,19,21,29,42,43,49,83,88-90 17 (Scheme 7).
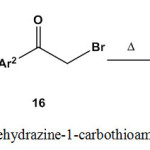 |
Scheme 7: Reaction of arylethylidenehydrazine-1-carbothioamides with 1-aryl-2-bromoethanone |
Also, refluxing of thiosemicarbazones 5, with heterocyclic moiety, with 1-aryl-2-substituted (unsubstituted)-2-bromoethanone 18 afforded 2-hydrazono-4-arylthiazoles49,52,55,74,76,80,84,88-93 19 (Scheme 8).
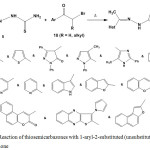 |
Scheme 8: Reaction of thiosemicarbazones with 1-aryl-2-substituted (unsubstituted)-2-bromoethanone |
Conversion of ethylidenehydrazine-1-carbothioamides 3 or 5 into 2-hydrazonothiazoles with coumarin moiety 21 was achieved through their reactions with 3-(2-bromoacetyl)-2H-chromen-2-one (20) in ethanol7,21,23,71,84,86,94,95 (Scheme 9).
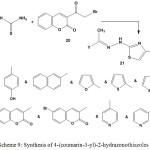 |
Scheme 9: Synthesis of 4-(coumarin-3-yl)-2-hydrazonothiazoles |
2-Arylhydrazono-5-(2-fluorophenyl)thiazoles 23 were synthesized from the reaction of 1-(1-arylethylidene)thiosemicarbazides 3 with a-bromoketone 22 in ethanolic solution containing triethylamine as a basic catalyst30 (Scheme 10)
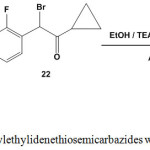 |
Scheme 10: Reaction of arylethylidenethiosemicarbazides with a-bromoketone |
Thiosemicarbazones 3 or 5 were reacted with 1,4-bis-(2-bromoacetyl)benzene (24) under thermal condition to give 1,4-phenylene-bis-thiazolyl derivatives13 25 (Scheme 11).
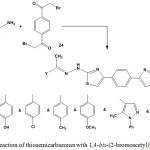 |
Scheme 11: Reaction of thiosemicarbazones with 1,4-bis-(2-bromoacetyl)benzene |
The reactivity of thiosemicarbazones 3 or 5 towards other a-haloketones was investigated. Thus, treatment of 3 or 5 with chloroacetone 26 furnished the corresponding 4-methyl-2-hydrazonothiazoles 276,10,19,42,53,78,80,83 (Scheme 12).
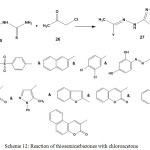 |
Scheme 12: Reaction of thiosemicarbazones with chloroacetone |
Reaction with Chloroethanoic acid or ethyl α-haloalkanoate
Cyclocondensation of ethylidenehydrazine-1-carbothiamides 3 or 5 with chloroethanoic acid (28) in ethanolic solution, containing anhydrous sodium acetate afforded thiazole-4(5H)-one derivatives7,8,26,45,57,60 29 (Scheme 13).
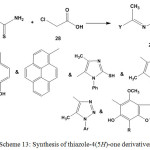 |
Scheme 13: Synthesis of thiazole-4(5H)-one derivatives |
Refluxing of chloroethanoic acid (28) with ethylidenehydrazine-1-carbothiamides 30a or 30b, in ethanolic solution containing sodium acetate, led to formation of tricyclic compounds [2,4,6,7-tetraazabicyclo[7.3.1]trideca-1(13),3,5,7,9,11-hexaen-5-yl]thioethanoic acid (33) or [2,4,6,7-tetraazabicyclo[7.2.2]trideca-1(11),3,5,7,9,12-hexaen-5-yl]thioethanoic acid (34), respectively96 (Scheme 14). The reactions proceeded by nucleophilic substitution and intramolecular condensation of amino group of thiourea residue and carbonyl of amide linkage.
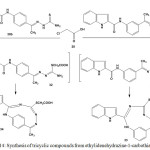 |
Scheme 14: Synthesis of tricyclic compounds from ethylidenehydrazine-1-carbothiamides |
Cyclocondensation of bis-oxyphenylthiosemicarbazones 35 with two equivalent of chloroethanoic acid (28) gave the respective bis-[2-(4-oxybenzylidene) hydrazono)-thiazol-4(5H)-one]26 36 (Scheme 15).
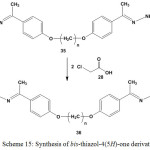 |
Scheme 15: Synthesis of bis-thiazol-4(5H)-one derivatives |
Treatment of ethylidenehydrazine-1-carbothioamide 5 with chloroethanoic acid or ethyl chloroethanoate gave 2-hydrazonothiazolidin-4-one72 (37) (Scheme 16).
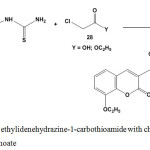 |
Scheme 16: Reaction of ethylidenehydrazine-1-carbothioamide with chloroethanoic acid or ethyl chloroethanoate |
Conduction of 1-(1-arylethylidene)thiosemicarbazides 3 with ethyl 2-chloro-2-phenylacetate (38) under reflux conditions gave the respective 2-[2-(1-arylethylidene) hydrazono]-5-phenyl-thiazol-4(5H)-ones16 39 (Scheme 17).
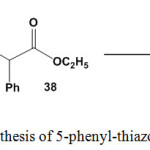 |
Scheme 17: Synthesis of 5-phenyl-thiazol-4(5H)-ones |
Similarly, reaction of ethylidenehydrazine-1-carbothioamides 3 or 5 with ethyl bromoethanoate (40) under reflux condition in an anhydrous potassium carbonate ethanolic solution afforded 4-thiazolidenones42,43,76,78,90,97 41(Scheme 18).
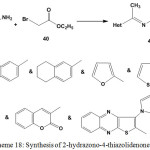 |
Scheme 18: Synthesis of 2-hydrazono-4-thiazolidenones |
Cyclocondensation of ethylidenehydrazine-1-carbothioamide 5 with ethyl bromoethanoate (40) in an equal molar ratio, in ethanolic solution containing catalytic amount of fused sodium acetate, afforded thiazole-5(4H)-one derivative 41. However, reaction of two moles of ethyl bromoethanoate with one mole of compound 5 gave N-ethoxycarbonylthiazole-5(4H)-one derivative6,53,78 42, which was obtained from treatment of 40 with 41 (Scheme 19)
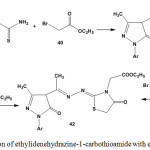 |
Scheme 19: Reaction of ethylidenehydrazine-1-carbothioamide with ethyl bromoethanoate |
Treatment of arylethylidenethiosemicarbazide 3 with ethyl 2-bromopropanoate (43) under reflux condition in absolute ethanol/piperidine mixture furnished 5-methyl-4-thiazolidenone43 44 (Scheme 20).
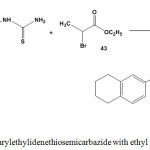 |
Scheme 20: Reaction of arylethylidenethiosemicarbazide with ethyl 2-bromopropanoate |
Reaction with Chloroacetonitrile
Reaction of chloroacetonitrile with 1-[1-(2-naphthyl)ethylidene]thiosemicarbazide (3) in ethanol/triethylamine mixture, under reflux condition, underwent cyclization to give the respective 2,5-dihydro-4-aminothiazole derivative42 45 (Scheme 21).
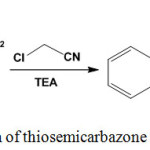 |
Scheme 21: Reaction of thiosemicarbazone with chloroacetonitrile |
Reaction with α-halo dicarbonyl Compounds
Refluxing of ethylidenehydrazine-1-carbothioamides 3 or 5 with a-halo dicarbonyl compounds such as; chloroacetylacetone (46), ethyl chloroacetoacetate (47), and chloroacetoacetanilide (48) gave the respective 2-hydrazono-4-methylthiazoles20,42,43,52,57 49a-c (Scheme 22).
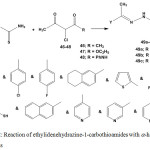 |
Scheme 22: Reaction of ethylidenehydrazine-1-carbothioamides with a-halo dicarbonyl compounds |
Conventional thermal heating or microwave irradiation of a mixture of ethylidenehydrazine-1-carbothioamides 3 or 5 and ethyl bromopyruvate (50) gave 4-ethoxycarbonylthiazole derivatives98 51 (Scheme 23).
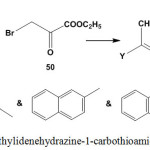 |
Scheme 23: Reaction of ethylidenehydrazine-1-carbothioamides with ethyl bromopyruvate |
Reaction with Dihalo Compounds
Treatment of ethylidenehydrazine-1-carbothioamide 5 with 2,3-dichloroquinoxaline (52) in absolute ethanol, under reflux condition gave ethylidenehydrazonothiazolo[5,4-b]quinoxaline7 53 (Scheme 24).
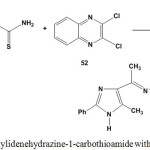 |
Scheme 24: Reaction of ethylidenehydrazine-1-carbothioamide with 2,3-dichloroquinoxaline |
Reaction with Aldehydes
Condensation of 1-[1-(9-arylidene-3-methyl-5-phenyl-6,7,8,9-tetrahydro-[1,2,4]triazolo[3,4-b] quinazolin-1(5H)-yl)ethylidene]thiosemicarbazide (5) with 2-chlorobezaldehyde (54) gave the respective Schiff’s base compound99 55 (Scheme 25).
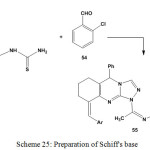 |
Scheme 25: Preparation of Schiff’s base |
Reaction with a,b-unsaturatednitrile Compounds
The response of ethylidenehydrazine-1-carbothioamide 5 towards different acrylonitrile derivatives was investigated52,57. Thus, reaction of 5 with arylidenemalononitriles 56, 2-cyano-N,3-diphenylacrylamide (57), and 3-aryl-2-cyano-prop-2-enethioamide (58), in dioxane under microwave irradiation52 or methanol under thermal conditions57 furnished the respective 1,3-thiazine derivatives52,57 60a-c (Scheme 26).
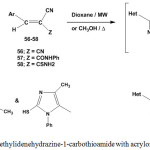 |
Scheme 26: Reaction of ethylidenehydrazine-1-carbothioamide with acrylonitrile derivatives |
Similarly, reaction of ethylidenehydrazine-1-carbothioamide 5 with ethoxymethylenemalononitrile (61) in refluxing methanol afforded 4-amino-2-hydrazono-1,3-thiazine-5-carbonitrile57 63 via non-isolable intermediate 62 (Scheme 27).
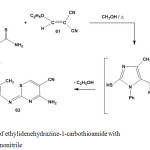 |
Scheme 27: Reaction of ethylidenehydrazine-1-carbothioamide with ethoxymethylenemalononitrile |
Treatment of an equimolar amounts of 2-[1-(2-pyridyl)ethylidene]hydrazine-1-carbothioamide (5) with arylmethylenemalononitriles 56 in pyridine solution gave 2-hydrazono-4-aryl-2,3-dihydrothiazole-5-carbonitriles100 64 (Scheme 28).
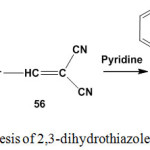 |
Scheme 28: Synthesis of 2,3-dihydrothiazole-5-carbonitriles |
On the other hand, reactions of 1-[1-(2-naphthyl)ethylidene]thiosemicarbazide (3) with benzylidenemalononitrile 56 or ethoxymethylenemalononitrile (61) afforded amino-pyrimidinethione derivative (67) or imino-pyrimidinethione derivative42 (68), respectively (Scheme 29).
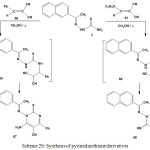 |
Scheme 29: Synthesis of pyrimidinethione derivatives |
A mixture of 2-arylhydrazono-4-amino-1,3-thiazine-5,6,6-tricarbonitriles 72 and 6-amino-2-thioxo-2,3-dihydropyrimidine-4,5-dicarbonitriles 73 was obtained101 from the reactions of thiosemicarbazones 3 or 5 with tetracyanoethylene (69) (Scheme 30).
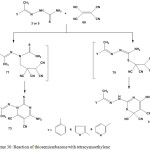 |
Scheme 30: Reaction of thiosemicarbazone with tetracyanoethylene |
Reaction with anhydrides
Cyclocondensation of ethylidenethiosemicarbazides 3 or 5 with acetic anhydride (74) led to formation of 3,5-di(N-acetylamino)-[1,3,4]thiadiazole derivatives59,102,103 77. The isolable products were formed via acetylation of primary nitrogen of thiourea residue (intermediate 75), intramolecular cyclization of thiol group into imino group (intermediate 76), and acetylation of NH group of [1,3,4]thiadiazole ring (Scheme 31).
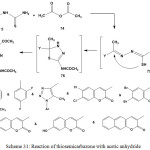 |
Scheme 31: Reaction of thiosemicarbazone with acetic anhydride |
Treatment of ethylidenehydrazine-1-carbothioamides 3 or 5 with maleic anhydride (78) gave 2-[2-hydrazono-4-oxo-4,5-dihydrothiazol-5-yl]ethanoic acid derivatives8,43 79 through thia-Michael reaction (Scheme 32).
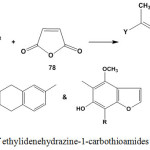 |
Scheme 32: Reaction of ethylidenehydrazine-1-carbothioamides with maleic anhydride |
Treatment of thiosemicarbazone (5), containing indole moiety, with maleic anhydride (78) or phthalic anhydride (80) in boiling ethanol gave 2-[2-hydrazono-4-oxo-4,5-dihydrothiazol-5-yl]ethanoic acid derivative (81) or N– substituted phthalimide derivative (82), respectively84 (Scheme 33).
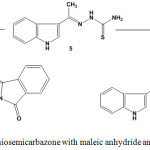 |
Scheme 33: Reactions of thiosemicarbazone with maleic anhydride and phthalic anhydride |
Reaction With Hydrochloric and Sulfuric Acids
Refluxing of thiosemicarbazone (5), containing benzofuran moiety, with methanol/hydrochloric acid mixture furnished [1,2,4]triazoline-3-thione derivative68 83 (Scheme 34).
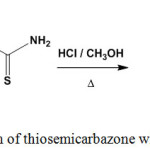 |
Scheme 34: Reaction of thiosemicarbazone with hydrochloric acid |
[1,2,4]Triazepino[6,5-c]quinolin-6(7H)-one (84) was prepared through dehydration of ethylidenehydrazine-1-carbothioamide (5), containing hydroxyquinolone moiety, by sulfuric acid at room temperature74 (Scheme 35).
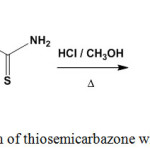 |
Scheme 35: Dehydration of ethylidenehydrazine-1-carbothioamide |
Reaction with dialkyl but-2-ynedioate
Refluxing of ethylidenehydrazine-1-carbothioamides 3 or 5 with dimethyl but-2-ynedioate or diethyl but-2-ynedioate in methanol afforded the respective alkyl 2-[2-hydrazono-4-oxothiazol-5(4H)-ylidene] ethanoate derivatives42,52,57,84,100,104,105 88a,b (Scheme 36).
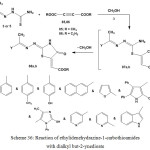 |
Scheme 36: Reaction of ethylidenehydrazine-1-carbothioamides with dialkyl but-2-ynedioate Click here to View scheme |
Self-Condensation Reaction
Microwave irradiation of ethylidenehydrazine-1-carbothioamides 3 or 5 led to self-condensation and give 3,4-diazahex-2,4-diene derivatives 8915 (Scheme 37).
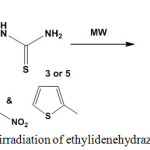 |
Scheme 37: Microwave irradiation of ethylidenehydrazine-1-carbothioamides |
Vilsmeier-Haack Reaction
Treatment of bis-thiosemicarbazones 90 with Vilsmeier-Haack reagent furnished the respective 6,8-bis-pyrazolylcoumarine derivative106 92 (Scheme 38).
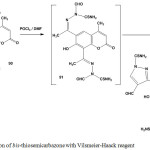 |
Scheme 38: Reaction of bis-thiosemicarbazone with Vilsmeier-Haack reagent |
Biological Activity
Antimicrobial Activity
Ethylidenethiosemicarbazide 3 or 5 were proclaimed to display a wide range of antibacterial and antifungal activities with different pharmacophore moieties4,6,7,25,39,58,64,65,67,68,72 (Chart 1).
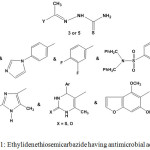 |
Graph 1: Ethylidenethiosemicarbazide having antimicrobial activity |
Antiviral Activity
Ethylidenethiosemicarbazide with aryl or benzimidazole substituents were evaluated as antiviral agents and showed moderate activity in most cases5,33,107,108 (Chart 2).
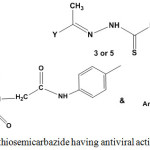 |
Graph 2: Ethylidenethiosemicarbazide having antiviral activity |
Anticancer Activity
Different alicyclic, aryl, or heterocyclic moieties introduced to thiosemicarbazone scaffolds 3 or 5 led to strengthen the anticancer activities against different cell lines1,7,18,31,47,48,51,73,75,85,96 (Chart 3).
 |
Graph 3: Ethylidenethiosemicarbazide having anticancer activity |
Anticonvulsant Activity
Thiosemicarbazones are promising anticonvulsant candidates8 that contain non-polar groups (aryl or heteroaryl) and thiourea residue (polar group which is responsible for hydrogen bonding) (Chart 4).
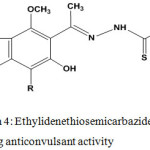 |
Graph 4: Ethylidenethiosemicarbazide having anticonvulsant activity |
Antiparasitic Activity
Recent research focuses on developing new drugs for Chagas diseases, caused by the protozoan parasite Trypanosoma Cruzi. Ethylidenethiosemicarbazides were evaluated and displayed higher activity against T. Cruzi9,10,24,38 (Chart 5).
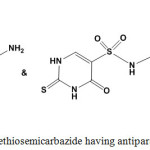 |
Graph 5: Ethylidenethiosemicarbazide having antiparasitic activity |
Miscellaneous
Other pharmaceutical applications of ethylidenethiosemicarbazides such as; Tyrosinase inhibitors51, antihypertensive66, antioxidant36, antiamoebic3, and antitubercular agents2 have been reported (Chart 6).
 |
Graph 6: Miscellaneous activities of Ethylidenethiosemicarbazide |
Acknowledgment
The authors represent their glory to King Abdulaziz University for funding our research project (grant No: 1-17-01-006-0001).
References
- dos Santos, T. A. R.; da Silva, A. C.; Silva, E. B.; de Moraes Gomes, P. A. T.; Espíndola, J. W. P.; de Oliveira Cardoso, M. V.; Moreira, D. R. M.; Leite, A. C. L.; Pereira, V. R. A. Biomed. Pharmacother. 2016, 82, 555-560.
CrossRef - Abhale, Y. K.; Shinde, A.; Deshmukh, K. K.; Nawale, L.; Sarkar, D.; Mhaske, P. C. Med. Chem. Res. 2017, 26, 2557-2567.
CrossRef - Iqbal, P. F.; Bhat, A. R.; Azam, A. Eur. J. Med. Chem. 2009, 44, 2252-2259.
CrossRef - Reis, D. C.; Despaigne, A. A. R.; Da Silva, J. G.; Silva, N. F.; Vilela, C. F.; Mendes, I. C.; Takahashi, J. A.; Beraldo, H. Molecules 2013, 18, 12645-12662.
CrossRef - Vitale, G.; Corona, P.; Loriga, M.; Carta, A.; Paglietti, G.; Giliberti, G.; Sanna, G.; Farci, P.; Marongiu, M. E.; La Colla, P. Eur. J. Med. Chem. 2012, 53, 83-97.
CrossRef - Bashandy, M. S. Asian J. Chem. 2011, 23, 3191-3201.
- Abdel-Wahab, B. F.; Awad, G. E. A.; Badria, F. A. Eur. J. Med. Chem. 2011, 46, 1505-1511.
CrossRef - Ragab, F. A.; El-Sayed, N. A. M..; Eissa, A. A. M.; El Kerdawy, A. M. Chem. Pharm. Bull. 2010, 58, 1148-1156.
CrossRef - Costa, L. B.; de Oliveira Cardoso, M. V.; de Oliveira Filho, G. B.; de Moraes Gomes, P. A. T.; Espíndola, J. W. P.; de Jesus Silva, T. G.; Torres, P. H. M.; Junior, F. P. S.; Martin, J.; Queiroz de Figueiredo, R. C. B.; Leite, A. C. L. Bioorg. Med. Chem. 2016, 24, 1608-1618.
CrossRef - de Oliveira Filho, G. B.; de Oliveira Cardoso, M. V.; Espíndola, J. W. P.; Oliveira e Silva, D. A.; Ferreira, R. S.; Coelho, P. L.; dos Anjos, P. S.; de Souza Santos, E.; Meira, C. S.; Moreira, D. R. M.; Soares, M. B. P.; Leite, A. C. L. Eur. J. Med. Chem. 2017, 141, 346-361.
CrossRef - Narkhede, H. I.; Nevagi, R. J.; Kumbhare, M.; Kaur, P. Der Pharm. Chem. 2014, 6, 221-227.
- Netalkar, P. P.; Netalkar, S. P.; Revankar, V. K. Appl. Organomet. Chem. 2015, 29, 280-289.
CrossRef - Abdelrazek, F. M.; Gomha, S. M.; Metz, P.; Abdalla, M. M. J. Heterocycl. Chem. 2017, 54, 618-623.
CrossRef - Gomha, S. M.; Ahmed, S. A.; Abdelhamid, A. O. Molecules 2015, 20, 1357-1376.
CrossRef - Chattopadhyay, G.; Ray, P. S. Synth. Commun. 2011, 41, 2607-2614.
CrossRef - Moreira, D. R. M.; Santos, D. S.; do Espirito Santo, R. F.; dos Santos, F. E.; de Oliveira Filho, G. B.; Leite, A. C. L.; Soares, M. B. P.; Villarreal, C. F. Chem. Biol. Drug Des. 2017, 90, 297-307.
CrossRef - Thanusu, J.; Kanagarajan, V.; Gopalakrishnan, M. Bioorg. Med. Chem. Lett. 2010, 20, 713-717.
CrossRef - Gomha, S. M.; Riyadh, S. M.; Mahmmoud, E. A.; Elaasser, M. M. Chem. Heterocycl. Compd. 2015, 51, 1030-1038.
CrossRef - Riyadh, S. M.; Deawaly, A. A.; Ahmed, H. E. A.; Afifi, T. H.; Ihmaid, S. Med. Chem. Res. 2017, 26, 1956-1968.
CrossRef - Makam, P.; Thakur, P. K.; Kannan, T. Eur. J. Pharm. Sci. 2014, 52, 138-145.
CrossRef - Chimenti, F.; Bizzarri, B.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Carradori, S.; D’Ascenzio, M.; Scaltrito, M. M.; Sisto, F. J. Heterocycl. Chem. 2010, 47, 1269-1274.
CrossRef - Makam, P.; Kankanala, K.; Prakash, A.; Kannan, T. Eur. J. Med. Chem. 2013, 69, 564-576.
CrossRef - Arshad, A.; Osman, H.; Bagley, M. C.; Lam, C. K.; Mohamad, S.; Zahariluddin, A. S. M. Eur. J. Med. Chem. 2011, 46, 3788-3794.
CrossRef - Blau, L.; Menegon, R. F.; Trossini, G. H. G.; Dutra Molino, J. V.; Vital, D. G.; Barretto Cicarelli, R. M.; Duó Passerini, G.; Bosquesi, P. L.; Chin, C. M. Eur. J. Med. Chem. 2013, 67, 142-151.
CrossRef - Jagadeesh, M.; Kumar, V. A.; Ramachandraiah, C.; Reddy, A. V. J. Appl. Pharm. Sci. 2013, 3, 111-115.
- Ayyash, A. N.; Jaffer, H. J.; Tomma, J. H. Am. J. Org. Chem. 2014, 4, 52-62.
- Saravanan, R. R.; Seshadri, S.; Gunasekaran, S.; R. Mendoza-Meroño, R.; Garcia-Granda, S. Spectrochim. Acta, Part A Mol. Biomol. Spect. 2015, 139, 321-328.
CrossRef - Ma, J.-X.; Lei, X.-F.; Wang, Y.-H.; Sun, Y.-Y. Z. Kristallograph. – New Crystal Str. 2013, 228, 453-454.
- Anbazhagan, R.; Sankaran, K. R. Spectrochim. Acta, Part A Mol. Biomol. Spect. 2015, 135, 984-993.
CrossRef - Pirbasti, F. G.; Mahmoodi, N. O.; Shiran, J. A. J. Sulfur Chem. 2016, 37, 196-210.
CrossRef - Akgemci, E. G.; Saf, A. O.; Tasdemir, H. U.; Turkkan, E.; Bingol, H.; Turan, S. O.; Akkiprik, M. Spectrochim. Acta, Part A Mol. Biomol. Spect. 2015, 136, 719-725.
CrossRef - Kilic-Cikla, I.; Guveli, S.; Bal-Demirci, T.; Aygun, M.; Ulkuseven, B.; Yavuz, M. Polyhedron 2017, 130, 1-12.
CrossRef - El-Sabbagh, O. I. Arch. Pharm. 2013, 346, 733-742.
CrossRef - Salem, M. A.; Thabet, H. K. H.; Helal, M. H.; Abdelaal, A. S.; Ammar, Y. A. Chem. Sci. J. 2011, 32, 1-12.
- Erol, I.; Sahin, Z.; Ozcan, L. Polymer Eng. Sci. 2013, 53, 1383-1393.
CrossRef - Bayoumi, W. A.; Elsayed, M. A.; Baraka, H. N.; Abou-Zeid, L. Arch. Pharm. 2012, 345, 902-910.
CrossRef - Salem, M. A. Org. Chem. Indian J. 2009, 5, 386-396.
- Fathalla, O. A.; Haiba, M. E.; Anwar, M. M.; Almutairi, M. S.; Maghraby, A. S.; Bahgat, M. M. Res. Chem. Intermed. 2013, 39, 2157-2185.
CrossRef - More, U. A.; Joshi, S. D.; Aminabhavi, T. M.; Gadad, A. K.; Nadagouda, M. N.; Kulkarni, V. H. Eur. J. Med. Chem. 2014, 71, 199-218.
CrossRef - Desai, N. C.; Joshi, V. V.; Rajpara, K. M.; Vaghani, H. V.; Satodiya, H. M. Med. Chem. Res. 2013, 22, 1893-1908.
CrossRef - El-Sabbagh, O. I.; Ibrahim, S. M.; Baraka, M. M.; Kothayer, H. Arch. Pharm. 2010, 343, 274-281.
- Gomha, S. M.; Badrey, M. G. J. Chem. Res. 2013, 86-90.
CrossRef - Haiba, M. E.; Abd El-Karim, S. S.; Gouhar, R. S.; El-Zahar, M. I.; El-Awdan, S. A. Med. Chem. Res. 2014, 23, 3418-3436.
CrossRef - Sharma, S.; Bedi, M.; Varshney, S.; Varshney, A. K. Indian J. Chem. Soc. 2012, 89, 41-50.
- Khalifa, N. M.; Mohamed, M. S.; Zaki, M. E.; Al-Omar, M. A.; Yasser M. Zohny, Y. M. Res. Chem. Intermed. 2014, 40, 1565-1574.
CrossRef - Gan, C.; Fan, L.; Huang, Y.; Liu, Z.; Cui, J. Med. Chem. 2013, 9, 846-854.
CrossRef - Gan, C.-F.; Liu, Z.-P.; Wei, W.-X.; Huang, Y.-M.; Cui, J.-G. Huaxue Yanjiu Yu Yingyong (Chem. Res. Appl.) 2013, 25, 647-654.
- Liao, L.; Jiao, Y.-X.; Yao, Q.-C.; Huang, Y.-M. Huaxue Shiji 2012, 34, 211-215.
- Hassan, A. A.; Ibrahim, Y. R.; El-Sheref, E. M.; Abdel-Aziz, M.; Brase, S.; Nieger, M. Arch. Pharm. 2013, 346, 562-570.
CrossRef - Tyagi, M.; Chandra, S.; Tyagi, P. Spectrochim. Acta, Part A Mol. Biomol. Spect. 2014, 117, 1-8.
CrossRef - Dong, H.; Liu, J.; Liu, X.; Yu, Y.; Cao, S. Bioorg. Chem. 2017, 75, 106-117.
CrossRef - Gomha, S. M.; Riyadh, S. M.; Mahmmoud, E. A.; Elaasser, M. M. Heterocycles 2015, 91, 1227-1243.
CrossRef - Abdelall, M. M. Phosphorus, Sulfur Silicon 2009, 184, 2208-2226.
CrossRef - Rana, A.; Parekh, N.; Dabhi, H.; Bhoi, D.; Kumari, N. E-J. Chem. 2011, 8, 1820-1831.
CrossRef - Rana, A. K.; Parekh, N. R.; Dabhi, H. R.; Nadkarni, S. S. E-J. Chem. 2009, 6, 747-752.
CrossRef - Chapkanov, A. Bulg. Chem. Ind. 2008, 79, 13-16.
- Gomha, S. M.; Riyadh, S. M.; Abbas, I. M.; Bauomi, M. A. Heterocycles 2013, 87, 341-356.
CrossRef - Eliazyan, K. A.; Knyazyan, A. M.; Pivazyan, V. A.; Ghazaryan, E. A.; Harutyunyan, S. V.; Yengoyan, A. P. J. Heterocycl. Chem. 2013, 50, 1083-1088.
- Wang, H.-C.; Li, R.-O.; Dong, H.-R.; Dong, H.-S. Indian J. Chem. 2010, 49B, 521-525.
- Wang, H.-C.; Li, R.-O.; Dong, H.-R.; Dong, H.-S. Indian J. Heterocycl. Chem. 2009, 19, 99-100.
- Ingale, A. P. J. Chem. Pharm. Res. 2014, 6, 460-464.
- Ismail, T.; Rossouw, D. D.; Beukes, P.; Slabbert, J. P.; Smith, G. S. Inorg. Chem. Commun. 2013, 33, 154-157.
CrossRef - Cobeljic, B.; Pevec, A.; Turel, I.; Spasojevic, V.; Milcic, M.; Mitic, D.; Sladic, D.; Andelkovic, K. Polyhedron 2014, 69, 77-83.
CrossRef - Lanjewar, K. R.; Ghatole, A. M.; Bhongade, R. P.; Gaidhane, M. K.; Rewatkar, S. B. World J. Pharm. Res. 2014, 3, 1445-1453.
- Nevagi, R. J.; Narkhede, H. I. Der Pharma Chim. 2014, 6, 135-139.
- Narkhede, H. I.; Nevagi, R. J.; Kumbhare, M.; Kaur, P. Der Pharm. Chem. 2014, 6, 221-227.
- Lanjewar, K. R.; Ghatole, A. M.; Gaidhane, M. K. Int. J. Pharm. Pharmecuit. Sci. 2013, 5, 601-603.
- Abdou, S. E.; El-Qusy, S. M.; Ghabrial, S. S.; Haggag, M. I. Modern Appl. Chem. 2011, 5, 140-149.
- Abdel-Gawad, H.; Mohamed, H. A.; Dawood, K. M.; Badria, F. A. Chem. Pharm. Bull. 2010, 58, 1529-1531.
CrossRef - Gomha, S. M.; Khalil, K. D. Molecules 2012, 17, 9335-9347.
CrossRef - Thota, S.; Nadipelly, K.; Shenkesi, A.; Yerra, R. Med. Chem. Res. 2015, 24, 1162-1169.
CrossRef - Mohamed, H. M.; Abd El-Wahab, A. H. F.; Ahmed, K. A.; El-Agrody, A. M.; Bedair, A. H.; Eid, F. A.; Khafagy, M. M. Molecules 2012, 17, 971-988.
CrossRef - Cushing, T. D.; Baichwal, V.; Berry, K.; Billedeau, R.; Bordunov, V.; Broka, C.; Cardozo, M.; Cheng, P.; Clark, D.; Dalrymple, S.; DeGraffenreid, M.; Gill, A.; Hao, X.; Hawley, R. C.; He, X.; Jaen, J. C.; Labadie, S. S.; Labelle, M.; Lehel, C.; Lu, P.-P.; McIntosh, J.; Miao, S.; Parast, C.; Shin, Y.; Sjogren, E. B.; Smith, M.-L.; Talamas, F. X.; Tonn, G.; Walker, K. M.; Walker, N. P. C.; Wesche, H.; Whitehead, C.; Wright, M.; Browner, M. F. Bioorg. Med. Chem. Lett. 2011, 21, 417-422.
CrossRef - Sankaran, M.; Kumarasamy, C.; Chokkalingam, U.; Mohan, P. S. Bioorg. Med. Chem. Lett. 2010, 20, 7147-7151.
CrossRef - Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Med. Chem. Res. 2013, 22, 267-277.
CrossRef - Geies, A. A.; Elossaily, Y. A.; Moustafa, O. S. Russ. J. Bioorg. Chem. 2012, 38, 526-532.
CrossRef - Gaber, A. M.; Geies, A. A. Afinidad LXVI 2010, 546, 154-159.
- Bedair, A. H.; Abd el-wahab, H. F.; El-agrody, A. M.; Ali, F. M.; Halawa, A. H.; El-Sherbiny, G. M. J. Serbian Chem. Soc. 2006, 71, 459-469.
CrossRef - Aly, A. S.; Abu-Zied, K. M.; Gaafar, A. M. Phosphorus, Sulfur Silicon Relat. Elem. 2008, 183, 3063-3078.
CrossRef - Abdelhamid, A. O.; Ismail, Z. H.; Abdel-Aziem, A. J. Chem. Res. 2007, 609-616.
CrossRef - Farghaly, T. A.; Abdallah, M. A.; Masaret, G. S.; Muhammad, Z. A. Eur. J. Med. Chem. 2015, 97, 320-333.
CrossRef - Gomha, S. M.; Zaki, Y. H.; Abdelhamid, A. O. Molecules 2015, 20, 21826-21839.
CrossRef - Abdelhamid, A. O; Gomha, S. M. Synth. Commun. 2017, 47, 1409-1414.
CrossRef - Abdelhamid, A. O; Gomha, S. M.; Kandeel, S. M. J. Heterocycl. Chem. 2017, 54, 1529-1536.
CrossRef - Gomha, S. M.; Edrees, M. M.; Faty, R. A. M.; Muhammad, Z. A.; Mabkhot, Y. Chem. Cent. J. 2017, 11:37.
CrossRef - Khidre, R. E.; El-Gogary, S. R.; Mostafa, M. S. J. Heterocycl. Chem. 2017, 54, 2511-2519.
CrossRef - Gomha, S. M.; Farghaly, T. A.; Sayed, A. R. J. Heterocycl. Chem. 2017, 54, 1537-1542.
CrossRef - Jadav, S. S.; Kaptein, S.; Timiri, A.; De Burghgraeve, T.; Badavath, V. N.; Ganesan, R.; Sinha, B. N.; Neyts, J.; Leyssen, P.; Jayaprakash, V. Bioorg. Med. Chem. Lett. 2015, 25, 1747-1752.
CrossRef - Secci, D.; Carradori, S.; Bizzarri, B.; Bolasco, A.; Ballario, P.; Patramani, Z.; Fragapane, P.; Vernarecci, S.; Canzonetta, C.; Filetici, P. Bioorg. Med. Chem. 2014, 22, 1680-1689.
CrossRef - Carradori, S.; Rotili, D.; De Monte, C.; Lenoci, A.; D’Ascenzio, M.; Rodriguez, V.; Filetici, P.; Miceli, M.; Nebbioso, A.; Altucci, L.; Secci, D.; Mai, A. Eur. J. Med. Chem. 2014, 80, 569-578.
CrossRef - Chimenti, F.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Carradori, S.; Yanez, M.; Orallo, F.; Ortuso, F.; Alcaro, S. Bioorg. Med. Chem. 2010, 18, 5715-5723.
CrossRef - Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; Carradori, S.; D’Ascenzio, M.; Yáñez, M.; Orallo, F. Chem. Med. Commun. 2010, 1, 61-72.
CrossRef - de Oliveira Cardoso, M. V.; Pessoa de Siqueira, L. R.; da Silva, E. B.; Costa, L. B.; Hernandes, M. Z.; Rabello, M. M.; Ferreira, R. S.; da Cruz, L. F.; Moreira, D. R. M.; Pereira, V. R. A.; Brelaz de Castro, M. C. A.; Bernhardt, P. V.; Leite, A. C. L. Eur. J. Med. Chem. 2014, 86, 48-59.
CrossRef - Salem, M. E.; Darweesh, A. F.; Mekky, A. E. M.; Farag, A. M.; Elwahy, A. H. M. J. Heterocycl. Chem. 2017, 54, 226-234.
CrossRef - Yusufzai, S. K.; Osman, H.; Khan, M. S.; Mohamad, S.; Sulaiman, O.; Parumasivam, T.; Gansau, J. A.; Noviany, N. J. Med. Chem. Res. 2017, 26, 1139-1148.
- Sweidan, K.; Sabbah, D. A.; Bardaweel, S.; AbuSheikha, G.; Al-Qirim, T.; Salih, H.; El-Abadelah, M. M.; Mubarak, M. S.; Voelter, W. Can. J. Chem. 2017, 95, 858-862.
CrossRef - D’Ascenzio, M.; Bizzarri, B.; De Monte, C.; Carradori, S.; Bolasco, A.; Secci, D.; Rivanera, D.; Faulhaber, N.; Bordon, C.; Jones-Brando, L. Eur. J. Med. Chem. 2014, 86, 17-30.
CrossRef - Carradori, S.; D’Ascenzio, M.; De Monte, C.; Secci, D.; Yanez, M. Arch. Pharm. 2013, 346, 17-22.
CrossRef - Dawoud, N. T. A. Chem. Sci. Trans. 2017, 6, 114-128.
- Aly, A. A.; Ishak, E. A.; Brown, A. B. J. Sulfur Chem. 2014, 35, 382-393.
CrossRef - Hassan, A.; Ibrahim, Y. R.; El-Sheref, E. M.; Brase, S. J. Heterocycl. Chem. 2016, 53, 876-881.
CrossRef - Gireesh, T.; Khan, G. M. Z.; Kamble, R. R. J. Indian Chem. Soc. 2011, 88, 1459-1463.
- De Monte, C.; Carradori, S.; Secci, D.; D’Ascenzio, M.; Guglielmi, P.; Mollica, A.; Morrone, S.; Scarpa, S.; Aglian, A. M.; Giantulli, S.; Silvestri, I. Eur. J. Med. Chem. 2015, 105, 245-262.
CrossRef - Darehkordi, A.; Saidi, K.; Mohammad Reza Islami, M. R. Arkivoc 2007, (i), 180-188.
- Hassan, A. A.; Ibrahim, Y. R.; Aly, A. A.; El‐Sheref, E. M., Yamamoto, T. J. Heterocycl. Chem. 2013, 50, 473-477.
CrossRef - Nagamallu, R.; Srinivasan, B.; Ningappa, M. B.; Kariyappa, A. K. Bioorg. Med. Chem. Lett. 2016, 26, 690-694.
CrossRef - Asthana, S.; Shukla, S.; Ruggerone, P.; Vargiu, A. V. Biochemistry 2014, 53, 6941-6953.
CrossRef - Asthana, S.; Shukla, S.; Vargiu, A. V.; Ceccarelli, M.; Ruggerone, P.; Paglietti, G.; Marongiu, M. E.; Blois, S.; Giliberti, G.; La Colla, P. Biochemistry 2013, 52, 3752-3764.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









