Zinc-Alginate Beads for the Controlled Release of Rifampici
Deepathomas1, 3, M.S Latha2,3 and K. Kurienthomas1
and K. Kurienthomas1
1Department of Chemistry, Bishop Moore College, Mavelikara, Kerala, India.
2Department of Chemistry, Sree Narayana College, Chengannur, Kerala, India.
3Department of Chemistry,Sree Narayana College, Kollam, Kerala, India.
Corresponding Author E-mail; lathams2014@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340146
The present study describes the preparation of a carrier system based on alginate and zinc and to evaluate its potential for the controlled release of rifampicin, the poorly water soluble drug using ionic gelation technique. The surface topology of the developed beads were analyzed by scanning electron microscopy and the crosslinking process was investigated by Fourier transform infrared spectroscopy .The pH dependent swelling properties of the beads were analyzed.Drug entrapment efficiency and in vitro drug release studies were also investigated. The invitro cytotoxicity and antibacterial activity were evaluated. Results indicated that the entrapment efficiency improves as the amount of polymer incresed. The developed beads showed a pH dependent swelling behaviour. The sustained release of drug was more prominent in pH 7.4 than 1.2. The MTT assay results proved the cell biocompatibility of the developed beads. The beads showed good antibacterial activity.
KEYWORDS:Alginate; Drug Delivery; Rifampicin; Cytotoxicity; Tuberculosis
Download this article as:| Copy the following to cite this article: Deepathomas D, Latha M. S, Kurienthomas K. Zinc-Alginate Beads for the Controlled Release of Rifampici. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Deepathomas D, Latha M. S, Kurienthomas K. Zinc-Alginate Beads for the Controlled Release of Rifampici. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42794 |
Introduction
Tuberculosis(TB) is a chronic pulmonary diseasecaused by mycobacterium tuberculosis. Rifampicin (RIF) is one of the effective first line drug for TB and isthe semi synthetic hydrazine derivative of rifampicin B.Major limitations of conventional tuberculosis therapy using RIF tablets are the longduration of treatment with frequent multiple dosage due to its short biological half-life, low stability and low water solubility. The continuous presence of RIF at high concentration above therapeutic range forprolonged period of time is associated with severe side effects such as renal failure,hepatotoxicity, fever, and thrombocytopeniaand antibodyformation1, 2. Development of drug delivery system that release the antimicrobial agent in a slow and sustained manner, could provide maximum therapeutic benefit whileminimizing the side effects.
Several studies have been made towards developing controlled drug delivery systems for antitubercular drugsto improve the therapeutic efficacy and minimize the sideeffects3.Many of these systemssuffer from problems like lower drug entrapment, highercost, safetyissues, use of toxic organic solvents and complicating preparation steps4, 5.Alginate based formulations attracted considerable attention due to its biocompatible nature6. Most of them use calcium as the cross linking metal. But calcium–alginate complexes were reported to show some disadvantages such as sensitivity towards the physiological environment and rapid release of encapsulated drug7.It was found that zinc ionspossess a superior binding capacity towards alginatethan calcium and which are more resistant to hydration8.Metal ions also have specific physiological functions in the body.Zinc is an important trace element, and possess key role in biomineralization, maintenance of membrane structure and its function and nucleic acid metabolism. It also possess antibacterial activity9.The antibacterial activity of zinc may also offer unique therapeutic applications.
The purpose of the present study is to prepare zinc alginate (Zn-ALG) beads as a carrier for poorly water insoluble drug RIF. The drug encapsulation efficiency and drug release profile were analyzed. The interaction between zinc alginate and RIF was analyzed. Antimicrobial activity of the obtained zinc alginate beads were studied against Staphylococcus aureus (S.aureus)and Escherichia coli(E.coli).The invitro cytotoxicity of the beads was tested using MTT assay.
Materials and Methods
Materials
Sodium alginate of medium viscosity (viscosity of 2% solution, 25°C ≈ 3500 cps, Sigma-Aldrich, London), Rifampicin (Himedia Laboratories, Nasik),Zinc chloride (Merck, Germany).
Preparation of Zn-ALG and RIF Loaded Zn-ALG Beads
Known amount of sodium alginate was dissolved in deionized water and stirred for 30 minutes to obtain homogenous solution. A volume of 10 mL of this solution was dropped into zincchloride solutionof definite concentration using a 25-G needle from 5 cm distance from the surface at room temperature.The droplets were kept in solution for 20 h. Then, Zn-ALG beads were separated from the solution, washed several times with distilled water and dried.
For preparing RIF loaded Zn-ALG beads, requiredweight of RIF was dispersed into sodium alginate solution and the same procedure was repeated.
Characterization Zn-ALG Beads
The morphology of Zn-ALG bead was characterized using scanning electron microscope (JEOL JSM-6390LA). Zn-ALG crosslinking and RIF-ALG interactions were analyzed by recording the Fourier transform infrared (FTIR) spectra of RIF, sodium alginate, Zn-ALG beads and RIF loaded Zn-ALG beads using Schimadzu FTIR model 1801 between 400 and 4000 cm-1 wavelength range.
Swelling
The degree of swelling was measured by suspending accurately weighed 100 mg of Zn-ALG beads in 10 ml solutions of pH 1.2 and 7.4 for 6 h. The increase in the weight of beads at predetermined time intervals were noted. The swelling degree was calculated using equation (1).Data were presented as mean ± standard deviation (SD) based on 3 independent measurements.
![]()
Where Ws the weight of swollen is samples and Wd is the initial weight of the beads.
Entrapment Efficiency of Zn-ALG Beads
About 100 mg of RIF loaded Zn-ALG beads were dispersed in phosphate buffer solution (100mL) of pH 7.4 and sonicated for 30 min to extract RIF completely from the beads .The concentration of extracted drug was analysed by measuring the absorbance at 475 nm, on PerkinElmer Lambda bio 40UV–VIS spectrophotometer by using the calibration curve constructed from a series of solutions with known drug concentration. The entrapment efficiency percentage (% EE) was calculated using equation (2)
![]()
In Vitro RIF Release Study
In order to understand the RIF release pattern,,in vitro release studies were carried out in simulated gastric fluid (SGF, pH 1.2) andsimulated intestinal fluid (SIF, pH 7.4) as per United States Pharmacopoeia standard10. About 30 mg of dried drug loaded beads were introduced into a dialysis bag of molecular weight cut off 12,000 Da and is incubated with 100 ml of the release medium. Release studies were conducted at 37 ◦ C in an incubated shaker at 100 rpm. About 5 ml of solution was taken at predetermined time intervals, and add equal amount of release medium at the same time.The RIF content in the solution was found by measuring the absorbance at 475 nm, using Perkin Elmer Lambda bio 40UV–VIS spectrophotometer. Data were presented as mean ± standard deviation (SD) based on 3 independent measurements.
In Vitro Cytotoxicity
The in vitro cytotoxicity of the Zn-ALG beads were analyzed by MTT assay using L929 fibroblast cell lines. The cells were cultured with DMEM containing 10% FBS and the cultured cell lines were remained at 37ºC in a humidified 5% CO2 incubator for 24 h. The medium in all the wells except the control, was then replaced with test solution and incubated at 37ºC in a humidified 5% CO2 incubator for specified time. There after 30 µl of MTT (5mg/ml) was introduced in to the wells and incubated at 37ºC in a humidified 5% CO2 incubator for 4 hours. Then the medium was removed and 100µl DMSO was added to dissolve the formazan crystals. The absorbance of the solution were measured at 570nm using micro plate reader to determine the optical density (OD). OD of control was obtained from the cells without sample dosing. Cell viability of the sample (%) was calculated using equation (3)
![]()
Evaluation of Antibacterial Activity
The antibacterial activity of Zn-ALG beads were studied against S. aureus and E.coli. Petriplates containing 20ml Muller Hinton Agar Medium were seeded with bacterial culture of E.coli and Staphylococcus aureus (growth of culture adjusted according to McFards Standard, 0.5%). Wells of around 10mm was bored by means of a well cutter and add the sample. The plates were incubated for 24 hours at 37°C. The activity was obtained by measuring the diameter of the zone of inhibition (in mm) formed around the well (NCCLS, 1993).
Statistical Analysis
All the experiments were carried out in triplicate and the data was exhibited as mean ± standard deviation. The error bars have been inserted in all the data points in the figures.
Results and Discussion
Preparation and Characterization
The Zn-ALG beads were prepared by ionotropic gelation technique.Spherical beads were formed due to the ionic interaction between the negatively charged carboxyl group of alginate and the positively charged zinc ions. The effects of process variables like drug-polymer ratio and concentration of the cross linking solution onthe drug entrapment efficiency was evaluated. It was noted that the entrapment efficiency improves with increase in the concentration of polymer. Concentration of metal ions is very critical in determining the shape of the bead. At lowconcentration below 5% metal chloride solution, spherical beads were not formed and onlyflakes were observed. At high concentration of metal ions irregular lumps were formed. This may due to the extensive cross linking of zinc ion with the guluronic acid component of ALG.Spherical beads with good entrapment efficiency were obtained at 1:4 drug-polymer ratio and 5% ZnCl2 solution.
Table 1: Optimization parameters for the preparation of Zn-ALG beads
| Formulation codes | Zn2+Concentration(%, v/v) | RIF:ALG | Drug entrapment efficiency %(%EE) |
| F1 | 2 | 1:4 | – |
| F2 | 5 | 1:4 | 35.48±2.85 |
| F3 | 10 | 1:4 | – |
| F4 | 2 | 1:2 | – |
| F5 | 5 | 1:2 | 26.98±2.78 |
| F6 | 10 | 1:2 | – |
| F7 | 2 | 1:1 | – |
| F8 | 5 | 1:1 | 27.5±2.05 |
| F9 | 10 | 1:1 | – |
The morphology of Zn –ALG beads were studied by recording their SEM images as shown in fig.1. In order to get a clear picture about the surface topography of the beads, the images were recorded at low and high magnifications.It can be seen that beads were spherical in shape with rough surface (fig 1 a). Some minor cracks can be seen on the surface of bead. This may due to the rupture during the electron beam irradiation.
 |
Figure 1: SEM image of Zn-ALG bead Click here to View figure |
The interaction between drug and polymer were studied by FTIR spectroscopy. Spectra of the RIF, Na ALG, Zn -ALG andRIF loadedZn-ALG beads were given in Fig.2. The characteristic peak at 3445 cm-1 in the spectra of the sodium alginate is indicative of stretching vibrations of OH bond.The bandsaround 1649 and 1446 cm−1 were assigned to asymmetric and symmetric stretching vibrations of carboxylic carbonyl groups .During zinc cross linking process there is a remarkable shift of this carbonyl stretching vibration to lower wave number (1649 to 1635 cm-1, 1446 to 1420cm-1) .This is due to the coordinate bond formation between ALG and zinc ions11. RIF gives a unique band at 1736cm-1 due to furanone ring structure. This band is also present in the spectra of RIF loaded Zn –ALG showing the successful encapsulation of RIF in Zn-ALG without any change in its chemical nature. This also indicate that there is no chemical interaction between the drug and polymer during encapsulation.
 |
Figure 2: FTIR of a) RIF b) Na ALG c) Zn – ALG d) RIF loaded Zn- ALG Click here to View figure |
Swelling Percentage
The swelling behaviour of Zn-ALG beads were investigated in simulated gastric fluid and intestinal fluid at room temperature as shown in Fig 3. Swelling behaviour of drug carrier has a profound effect on the mechanism and kinetics of drug release. The swelling percentage was low at pH 1.2.This is due to the formation insoluble alginic acid as a result of protonation of carboxylate group. This reduces electrostatic repulsion among the carboxylate groups and minimize the degree of swelling.The strong hydrogen bond interactions also help to maintain the three dimensional network structure and hence reduce the water uptake. At this pH the system was stable and does not undergo any type of disintegration.
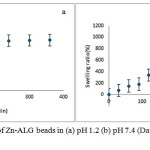 |
Figure 3: Swelling behaviour of Zn-ALG beads in (a) pH 1.2 (b) pH 7.4(Data presented as mean ± SD, n = 3) Click here to View figure |
At pH 7.4 a profound swelling isobserved.This is due to the anionic nature of ALG. At this pH the extent of ionization of carboxylate group of ALG increases, thus produces more carboxylate ions and these negatively charged ions repel each other and causes a rapid relaxation in ALG network. This relaxation enhances the inclusion of water molecule in to the bead network structure.
In Vitro Drug Release Study
Fig 4showed the release behaviour of RIF from Zn-ALG beads in SGF (pH 1.2) and SIF (pH 7.4).Approximately 10% of the drug was released in SGF over a period of 6 h. The pKa value of ALG is 3.6.Below this value, it is converted into alginic acid and form a tight three dimensional network. Zinc is also less selective towards alginate and can form a strong three dimensional network and retain the drug inside the bead.
At alkaline conditions, the pKa value is higher than 3.6, then the negatively charged carboxylate ions repel each other. As a result the three dimensional network relaxes and fluid can penetrate in to the Zn-ALG network. So RIF releaseis higher and almost 80% of the entrapped RIF was releasedin 6 h. The formation of gel diffusion layer help to hinder the outward transport of RIF, and maintain sustained release.This in vitro drug release study showed that the Zn-ALG system give a pH dependent drug release.
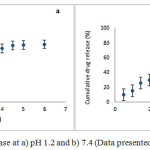 |
Figure 4: Cumulative RIF release at a) pH 1.2 and b) 7.4 (Data presented as mean ± SD, n = 3) Click here to View figure |
In vitro Cytotoxicity
Cytotoxicity evaluation is very important for a drug carrier since it is applied inside the body. MTT is usually used to measure the cytotoxicity. In this method the cytotoxicity is expressed in terms of cell viability. The cell viability of control is 100%. As seen in Fig.5, the L929 cell viability after being treated with the Zn -ALG was more than 80% even when the concentration was up to 100 µg/ml, indicating the drug carrier did not have significant cytotoxicity and possess excellent biocompatibility.
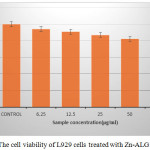 |
Figure 5: The cell viability of L929 cells treated with Zn-ALG Click here to View figure |
Evaluation of Antibacterial Activity
The antibacterial activity of Zn-ALG bead was analyzed by measuring the bacterial growth inhibition zone against S.aureus (gram positive bacterium) and E.coli (gram negative bacterium). The zone of inhibition (100µg/ml) for S.aureusand E.coli was 29mm and 32mm respectively. These observations clearly indicate that the obtained bead shows antibacterial activity against both gram positive and gram negative bacteria. The antibacterial activity of Zn2 can be explained on the basis of its ability to form bond with imidazole, thiole, carboxyl and amino group present in the bacterial protein. As a result some structural changes may occur and this hinders the transport through the plasma membrane and results in cell death12.
 |
Figure 6: Photograph of antimicrobial test results of Zn-ALG against a) S.aureus and b) E.coli Click here to View figure |
The prepared Zn-ALG beads showed sustained RIF release, biocompatible in nature and can effectively inhibit the growth of both S.aureus and E.coli. The in vitro studies suggest that the biocompatible drug delivery systemscan be used for sustained release of other drugs, enzymes, genomic materials and proteins.Further investigation using in vivo models should be performed to substantiate the results obtained from the in vitro studies.
Acknowledgements
We acknowledge the University Grants Commission (FIP/12th plan/KLKE002TF05) for the financial support.
Conflict of Interest
The Authors declare no conflict of interest.
References
- Ribeiro,A.C.; Rocha, A.; Soares,R.M.D.; Fonseca, L.P.; da Silveira,N.P.Carbohydr. Polym.2017, 157,267–274.
CrossRef - Amarnath Praphakar,R.; Munusamy,M.A.; Sadasivuni,K.K.; Rajan, M.Int. J. Pharm. 2016,513, 628–635.
CrossRef - He, D.; Deng, P.; Yang, L.; Tan, Q.; Liu, J.; Yang, M.; Zhang, J .Colloids Surfaces B Biointerfaces. 2013,103, 580–585.
CrossRef - Silveira, N.; Longuinho,M.M.; Leitão, S.G.; Silva, R.S.F.; Lourenço, M.C.; Silva, P.E.A.; Pinto, M.D.C.F.R.; Abraçado, L.G.; Finotelli, P.V. Mater. Sci. Eng. C. 2016 ,58 ,458–466.
CrossRef - Labuschagne, P.W.; Adami, R.; Liparoti, S.; Naidoo, S.; Swai, H.; Reverchon, E.J. Supercrit. Fluids.2014 , 95 , 106–117.
CrossRef - Goh, C.H .; Heng, P.W.S.; Chan, L.W.Carbohydr. Polym.2012, 88, 1–12.
CrossRef - Lacerda, L.; Parize, A.L.; Fávere,V.; Laranjeira, M.C.M.; Stulzer, H.K.Mater. Sci. Eng. C.201439 161–167.
- Assifaoui,A.; LoupiacC.; Chambin,O.; Cayot, P.Carbohydr. Res. 2010,345, 929–933.
CrossRef - Pati, R.; Sahu,R.; Panda, J.; Sonawane, A.Sci. Rep.2016 , 6 ,24184-24198.
CrossRef - U.S.P. Convention, US Pharmacopeia, 2002 ,XXV,2159.
- Raut,N.S.; Deshmukh,P.R.; Umekar,M.J.; Kotagale, N.R.Int. J. Pharm. Investig.2013 ,3,194–202.
CrossRef - Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš,I.B.; Raičević,S. Appl. Surf. Sci.2010, 256, 6083–6089.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









