Using Thermolytic Solution of Anionic-decorated Graphene Quantum Dots (GQDs) as Fluorescence Turn on-off Sensor for Selective Screening Test of Metal Ions
Khanitta Saenwong1, Chawangorn Putthasehn1, Atipa Tunsawat1, Prawit Nuengmatcha2 and Saksit Chanthai1
1Materials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand.
2Nanomaterials Chemistry Research Unit, Department of Chemistry, Faculty of Science and Technology, Nakhon Si Thammarat Rajabhat University, Nakhon Si Thammarat 80280, Thailand.
Corresponding Author E-mail: sakcha2@kku.ac.th
DOI : http://dx.doi.org/10.13005/ojc/340106
This research was aimed to prepare graphene quantum dots (GQDs) by thermolysis of citric acid using paraffin oil bath, preferably regarding weight of citric acid and heating temperature. Fluorescence spectrum of the blue GQDs solution (under UV light), with excitation lmax 365 nm was characterized. The fluorescence intensity at lmax 460 nm was used to calibrate their uniformity. The as-prepared GQDs were achieved with 0.25 g citric acid at 225°C for 5 min. The GQDs solution at pH 6-13 gave high fluorescence intensity without any effect of ionic strength and masking agent. Effect of some anionic salts using their corresponding mineral acids (1%, w/w) to decorate the GQDs was investigated. The results showed that each of HCl, HNO3 and H3PO4 enhances its fluorescence intensity of the GQDs decorated with Cl-, NO3-& PO43-, respectively, while the matrix effect of ClO4-, SO42- and BO33-gave very large extent of the quenching effect compared with that of bare GQDs. In addition, these anionic–decorated GQDs were applied for selective screening test with various metal ions. It was found that each kind of these anions shows strong intrinsic quenching and enhancing of their fluorescence intensity of the GQDs, suggesting further trends in their fluorescence determination of some metal ions.
KEYWORDS:Fluorescence Sensor; Metal Ions; Graphene Quantum Dots; Thermolysis; Citric Acid; Mineral Acids
Download this article as:| Copy the following to cite this article: Saenwong K, Putthasehn C, Tunsawat A, Nuengmatcha P, Chanthai S. Using Thermolytic Solution of Anionic-decorated Graphene Quantum Dots (GQDs) as Fluorescence Turn on-off Sensor for Selective Screening Test of Metal Ions. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Saenwong K, Putthasehn C, Tunsawat A, Nuengmatcha P, Chanthai S. Using Thermolytic Solution of Anionic-decorated Graphene Quantum Dots (GQDs) as Fluorescence Turn on-off Sensor for Selective Screening Test of Metal Ions. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42113 |
Introduction
It is well known that graphene has found widespread applications in many diverse fields in physics, chemistry, material science, and biology. It has been shown that when a graphene sheet is small enough, its properties can vary significantly. At a size less than 100 nm, a new kind of materials called graphene quantum dots (GQDs) is produced1. GQDs usually contain one or two layers of graphene with a size distribution mainly in the range of 3-20 nm2. GQDs are highly luminescent as a result of the quantum confinement and edge effects3. The amounts of oxygen-containing groups, structural defects, and doping elements also contribute considerably to their luminescence properties4. They have higher surface area, larger diameter, and better surface grafting properties compared with the conventional quantum dots (QDs) or carbon dots (CDs)5. In addition, GQDs show excellent water solubility, low toxicity, high stability, and good biocompatibility. They are considered to be a promising material to replace the commonly used semiconductor nanocrystals for a number of biosensing and bioimaging related applications6-8.
Glycine (GLY) functionalized graphene quantum dots (GLY-GQDs) by a simple and green pyrolysis method employing ethylene glycol as carbonsource, GLY as functional molecule was reported9. The as-synthesized GLY-GQDs exhibited excellent water solubility with about 22% fluorescence quantum yield. The fluorescence of GLY-GQDs was intensively quenched by Ce4+via forming non-luminescent complexes of GLY-GQDs-Ce4+in the presence of ascorbic acid. GQDs were also prepared by a simple carbonization method10. It was found that Ce4+could oxidize GQDs to produce a relatively intense chemiluminescence (CL) emission. It was attributed to the radiative recombination of oxidant-injected holes and thermally excited electrons in the GQDs. In order to show the analytical application potential of GQDs-Ce4+CL system, it was applied to the determination of uric acid. Simple fluorescent probe to detect ascorbic acid based on “off-to-on” mechanism of GQDs in the presence of Cu2+ion was developed11. The fluorescence of GQDs was greatly quenched by Cu2+. Upon addition of ascorbic acid, Cu2+was reduced to Cu+and the interaction of Cu2+-GQDs was destroyed, which inhibited the quenching and thus leading to fluorescence recovery of GQDs. An effective approach to produce GQDs was developed12, which based on the cutting of graphene oxide (GO) powder into smaller pieces and being reduced by a green approach, using sodium polystyrene sulfonate as a dispersant and L-ascorbic acid as the reducing agent, which is environmentally friendly. Then, the as-prepared GQDs were further used for the detection of heavy metal ions, Pb(II).
A facile method for the highly sensitive and selective sensing of biothiols based on GQDs was also developed13. They reported that GQDs emitted strong blue fluorescence in an aqueous buffer solution. It was observed that Hg(II) could efficiently bind with GQDs and quench its fluorescence intensity. When the biothiol compounds (glutathione, cysteine, or homocysteine) were added to the assay mixture of GQDs and Hg(II), it bound with Hg(II). Hg2+–GQD complex dissociated, and the fluorescence turn-on signal was detected.Hormozi-The as-prepared carbon dots (CDs) contain phenol groups on their surfaces was reported14, and due to the special coordination interaction of phenol groups with Fe3+ ions, this sensing system exhibits excellent sensitivity and selectivity toward Fe3+. In order to detect Fe2+ ions as well, a specific amount of H2O2 was introduced to the detection system to transform Fe2+ to Fe3+ of which the later one is the determinable form of iron. The boron-doped graphene quantum dots (B-GQDs) was synthesized15. The fluorescence intensity of the B-GQDs has been found to be very sensitive to the presence of Fe3+in the system but not to other possible coexisting metal ions, showing the high selectivity of this Fe3+ assay and highlighting that there is no need for tedious procedures to separate Fe3+ from other metal ions prior to the assay. Sulfur-doped graphene quantum dots (S-GQDs) with stable blue-green fluorescence by one-step electrolysis of graphite in sodium p-toluenesulfonate solution was synthesized16. Doping with S atoms improved the electronic properties and surface chemical reactivities of GQDs, which was beneficial for chemically binding with Fe3+forming S-GQDs-Fe3+. The S-GQDs emitting strong blue-green fluorescence were used as a novel fluorescence probe for the highly sensitive and selective detection of Fe3+. New class of dopamine-functionalized GQDs (DA-GQD) using one-step EDC/NHS coupling reaction for Fe3+detection was synthesized17. The functionalization between -COOH group of GQD molecule and amine group of dopamine results in the strong complexation interaction between catechol moiety of DA-GQD and Fe3+. The further oxidation of dopamine by Fe3+enhances the sensitivity and selectivity of sensing probes.The synthesis of D-penicillamine functionalized graphene quantum dots (DPA-GQD) through the thermal pyrolysis of citric acid and D-penicillamine was reported18. The resulting functionalized GODs exhibit ultra-high fluorescence intensity and sensitive and ultra-selective response to Fe3+. The nanosensor based on the GODs was developed for fluorescent detection of Fe3+in the iron supplement oral liquids.
This research study was aimed toward insights for preparation of GQDs via simple thermolysis of citric acid, and to well develop multi-functionalized GQDs by simple decoration with some anionic salts during thermolysis, expecting it would serve indirectly as potential doping carbon based material. Effects of experimental parameters including solution pH, weight of citric acid, heating temperature, and various kinds of anionic salts associated with GQDs affecting their fluorescence intensity were also optimized. Then, the as-prepared and decorated GQDs were preliminary subject to perform selective analysis of various metal ions in aqueous solution.
Materials and Methods
Chemicals
All chemicals used were AR grade from QRecTM (New Zealand) including citric acid, sodium hydroxide, sulfuric acid, perchloric acid, boric acid, hydrochloric acid, nitric acid, phosphoric acid, acetic acid, sodium acetate. Potassium dihydrogenphosphate and dipotassium hydrogen phosphate were from LOBA (India). Paraffin oil was purchased from Sigma-Aldrich (USA).
Instruments
Fluorescence Spectrophotometer, Shimadzu, RF-6000(Japan) was mainly used.pH meter, Model Proline B21, Becthai Equipment & Chemical (Thailand), analytical balance, Model BSA224S-CW, Scientific Promotion (Thailand), quartz cell, Fisher Scientific (USA) were also used. Round bottom flask, Pyrex® (England) and hot plate with magnetic stirrer in association with paraffin oil bath were also needed for this experiment.
Preparation of Gqds by Thermolysis of Citric Acid
Effect of Weight of Citric Acid
Since thermolysis of solid citric acid depends on its starting weight and preheated temperature with short synthetic time, various weights of citric acid (0.25, 0.5 and 0.75 g) were used to fuse in round bottle flask which was equilibrated at 200 °C in preheated paraffin oil bath for 5 min. Then, the melted citric acid was solubilized with 50 mL of 0.25 M NaOH solution while continuous magnetic stirring for 30 min at room temperature9. The obtained GQDs solution was diluted to a series of 10-50 mg/L, based on the original weight of citric acid used, prior to fluorescence measurement with excitation and emission wavelengths of 365 and 460 nm, respectively. Their fluorescence intensities were plotted with respect to the GQDs concentrations, giving calibration curves with different slopes.
Effect of Heating Temperature using Paraffin oil Bath
Generally, thermolysis process is required an appropriate heating temperature in accordance with heating time due to such colligative properties of substance. Thus, in this case, the preheating temperature at 200, 225 and 250 °C was approximately (10°C more or less) set up using the paraffin oil bath using 0.25 g citric acid under the same synthetic route and fluorescence measurement as mentioned above.
Effect of Solution pH
The 1.0 M acetate buffer solution was prepared and adjusted to appropriate pH 2-5 with HCl or NaOH solutions using pH meter. 1.0 M phosphate buffer solution was prepared and also adjusted to pH 6-13in the same manner.Then, 100 mg/L GQDs solution was subject to buffering capacity at pH range of 2-13 with 1.0 mL of those buffering solutions in 10.0 mL volumetric flask prior to fluorescence measurement.
Effects of NaCl and EDTA Concentrations
One mL of various concentrations of 0.05, 0.10, 0.25, 0.20 and 0.25 M NaCl was added into 100 mg/L GQDs solution and adjusted to 10 mL in volumetric flask prior to fluorescence measurement. In the same manner, 1.0 mL of 0.01, 0.02, 0.03, 0.04and 0.05 M EDTA was added into the GQDs solution and recorded their fluorescence spectra.
Effects of Anionic salts (1% w/w) Doping on GQDs
Following the suitable synthetic procedure of GQDs as mentioned above, some representative mineral acids (HCl, HClO4, HNO3, H2SO4, H3PO4, H3BO3), of 1% w/w were used and simultaneously added into the melted citric acid within 5 min during thermolysis step, then solubilized in 50 mL of 0.25 M NaOH in brown capped glass bottle with continuous magnetic stirring for 30 min at room temperature prior to fluorescence measurement using 100 mg/L of the mineral acids doped GQDs solution.
Selectivity Test of the Mineral Acids Doped GQDs on Various Metal Ions
One mL of 10 µM each solution of Na+, Mg2+,Ag+, Cu2+, Pb2+, Mn2+, Hg2+,Fe2+, Fe3+and Al3+ was added into each of various mineral acids doped GQDs solution (100 mg/L) and adjusted final volume to 10 mL with deionized water prior to measurement of fluorescence spectrum.
Results and Discussion
Synthesis and Fluorescence Characteristics Of GQDs
Graphene quantum dots (GQDs) were synthesized via thermolysis of solid citric acid using pre-heated paraffin oil bath for 5 min after its fusion, and were solubilized with 50 mL of 0.25 M NaOH. Citric acid weights of 0.25, 0.50 and 0.75 g were subject to melt in the pre-heated oil bath at 200°C for 5 min following the procedure mentioned above. Each of stock GQDs solutions were diluted approximately ranging between 10-50 mg/L. The fluorescence spectrum of GQDs solution was recorded between 400-600 nm showing emission lmax 460 nm with excitation lmax 365 nm (data not shown). Their calibration curves were constructed comparatively as shown in Fig. 1. It was found that the calibration curve: y = 179.41x – 11.43 with highest slope, even it was slightly larger standard deviations compared to others, was obtained from using 0.25 g citric acid. While those of calibration curves gave rather lower probably due to massive dependence on short thermolysis of citric acid at 200°C within 5 min. The effect of heating temperature (200, 225 and 250°C) of paraffin oil bath on calibration curve of GQDs solution was also investigated in the same manner using0.25 g citric acid as shown in Fig. 2.It was found that both slopes of two calibration curves (y = 213.06x – 263.32 and y = 227.62x – 400.42) showed no difference and were higher than that of the other one, considerably indicating that thermolysis of 0.25 g citric acid at heating temperature between 200 °C and 225 °C was comparable, while at 250°C it is too hot for rapid transformation of GQDs.
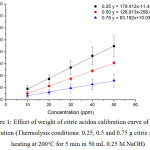 |
Figure 1: Effect of weight of citric acidon calibration curve of GQDs solution (Thermolysis conditions: 0.25, 0.5 and 0.75 g citric acid, heating at 200°C for 5 min in 50 mL 0.25 M NaOH) Click here to View figure |
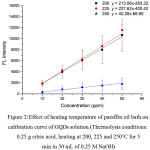 |
Figure 2:Effect of heating temperature of paraffin oil bath on calibration curve of GQDs solution (Thermolysis conditions: 0.25 g citric acid, heating at 200, 225 and 250°C for 5 min in 50 mL of 0.25 M NaOH) Click here to View figure |
Effects of pH, Ionic Strength and Masking Agent on Fluorescence Intensity of GQDs
Since thermolysis of citric acid to prepare GQDs was processed in atmosphere pressure, the chemical transformation of GQDs had been possessed with common functional groups like hydroxyl, epoxy and carboxyl parts. So, after solubilization of GQDs in 0.25 M NaOH, its fluorescence spectrum could be indicated. However, when GQDs were buffered with 0.1 M acetate buffer solution pH 2-5, there was no fluorescence intensity at all, acidic pH quenching effect pronounced. If GQDs were prepared in neutral to base buffer solution pH 6-13, their fluorescence intensities were recovered almost constantly as shown in Fig. 3.
In addition, effects of ionic strength as 0.05 0.10 0.15 0.20 and 0.25 M NaCl solutions and masking agent as 0.01, 0.02, 0.03, 0.04 and 0.05 EDTA solutions tested were also investigated (data not shown). It was found that both ionic strength and masking agent had no effect on the fluorescence intensity of the GQDs solution. It is further aimed that modification of the ionic strength of the mixture solution could be done in association with addition of EDTA to mask any metal ions interferences in sample.
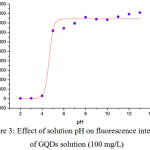 |
Figure 3: Effect of solution pH on fluorescence intensity of GQDs solution (100 mg/L) Click here to View figure |
Effect of Anionic Salts Decorating on GQDs During Thermolysis of Citric Acid
Basically, an element doping on GQDs during simple thermolysis of citric acid, using paraffin oil bath at 225 °C for 5 min and solubilized in 50 mL 0.25 M NaOH, could achieved as multi-functional carbon based materials for tuning fluorescence turn-on/off sensor probe for chemical detection. In this study, six kinds of mineral acids including hydrochloric acid, sulfuric acid, nitric acid, orthophosphoric acid, perchloric acid, and boric acid were introduced to the synthesis of GQDs. As shown in Fig. 4, two groups of fluorescence spectra were found, indicating that these anionic decorated GQDs solutions had different photoluminescence after excited at the same maximum excitation wavelength of 365 nm, resulted nearly in the same maximum emission wavelength of 460 nm. This means that these decorated GQDs exhibit simply the same fluorescence mechanism compared to that of undecorated GQDs. The enhancing effects of fluorescence intensity of the element decorated GQDs were found with three kinds of the anionic salts like Cl–, NO3– and PO43-, while their quenching effects were found with thermolysis of ClO4–, SO42-, and BO33-. Thus, trends in useful information for fluorescence turn-on/off sensor probe can be drawn from these results, if it is needed for various metal ions determination in any sample.
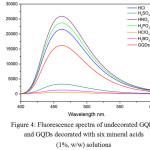 |
Figure 4: Fluorescence spectra of undecorated GQDs and GQDs decorated with six mineral acids (1%, w/w) solutions Click here to View figure |
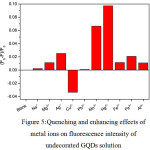 |
Figure 5: Quenching and enhancing effects of metal ions on fluorescence intensity of undecorated GQDs solution |
From Fig. 5, the fluorescence intensity of undecorated GQDs solution was normally obtained.9 When 1.0 mL each of 10 µM of Na+, Mg2+, Ag+, Cu2+, Pb2+, Mn2+, Hg2+, Fe2+, Fe3+ and Al3+ was mixed with the GQDs 100 mg/L in final volume of 10 mL, the quenching effects of Hg2+ and Mn2+were quite strong. But only Cu2+ gave slightly enhancing one, while other cations did not show much change in their quenching effects. So, selective and sensitivity test for Hg2+ and Mn2+ can be done, while optimization conditions for the sensitivity test for fluorescence turn-on sensor of Cu2+ concentration would be further investigated.
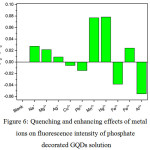 |
Figure 6: Quenching and enhancing effects of metal ions on fluorescence intensity of phosphate decorated GQDs solution |
From Fig. 6, the fluorescence intensity of phosphate decorated GQDs solution was increased higher than that of undecorated GQDs solution. When 1.0 mL each of 10 µM of Na+, Mg2+, Ag+, Cu2+, Pb2+, Mn2+, Hg2+, Fe2+, Fe3+ and Al3+ was mixed with the phosphate – GQDs 100 mg/L in final volume of 10 mL, the quenching effects of Hg2+ and Mn2+ were strongly comparable, while Fe2+ and Al3+ gave pronouncedly enhancing one.Other cations did not show much change in their quenching effects compared with that of blank (phosphate – GQDs without cation) solution. Optimization conditions for the selectivity and sensitivity test for fluorescence turn-off sensor for Hg2+ and Mn2+concentrations would be further checked. In addition, that for fluorescence turn-on mode for Fe2+ and Al3+ could be also investigated for further analysis.
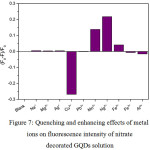 |
Figure 7: Quenching and enhancing effects of metal ions on fluorescence intensity of nitrate decorated GQDs solution Click here to View figure |
From Fig. 7, the fluorescence intensity of nitrate decorated GQDs solution was drastically increased higher than that of undecorated GQDs solution, giving the same pattern as found in Fig. 9. When 1.0 mL each of 10 µM of Na+, Mg2+, Ag+, Cu2+, Pb2+, Mn2+, Hg2+, Fe2+, Fe3+ and Al3+ was mixed with the nitrate – GQDs 100 mg/L in final volume of 10 mL, the quenching effects of Hg2+ was strongly obtained. The second one was Mn2+ which gave slightly quenching one, while other cations showed no change in their fluorescence intensities, except Cu2+ showed the enhancing effect drastically compared with that of blank (nitrate – GQDs without cation) solution. Optimization conditions for the selectivity 2and sensitivity test of Hg2+and Mn2+concentration would be further studied. In addition, selectivity test for Cu2+ using the N doped GQDs could be of interesting subject.
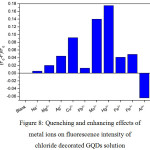 |
Figure 8: Quenching and enhancing effects of metal ions on fluorescence intensity of chloride decorated GQDs solution Click here to View figure |
From Fig. 8,the fluorescence intensity of chloride decorated GQDs solution was also higher than that of undecorated GQDs solution, giving the same pattern as found in Fig. 6 and Fig. 7. When 1.0 mL each of 10 µM of Na+, Mg2+, Ag+, Cu2+, Pb2+, Mn2+, Hg2+, Fe2+, Fe3+ and Al3+ was mixed with the chloride – GQDs 100 mg/L in final volume of 10 mL, the quenching effect of Hg2+ was strongly obtained. The second one was Mn2+ which gave slightly quenching one, while other cations showed rather changes in their fluorescence intensities compared with that of blank (chloride – GQDs without cation) solution. Optimization conditions for the selectivity and sensitivity test of Hg2+and Mn2+concentrations would be further studied. In addition, selective determination of Al3+ using Hg2+ quenched chloride – GQDs may be choice of further study.
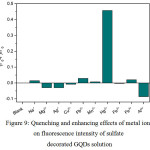 |
Figure 9: Quenching and enhancing effects of metal ions on fluorescence intensity of sulfate decorated GQDs solution Click here to View figure |
From Fig. 9, the fluorescence intensity of sulfate decorated GQDs solution was drastically less than that of undecorated GQDs solution. When 1.0 mL each of 10 µM of Na+, Mg2+, Ag+, Cu2+, Pb2+, Mn2+, Hg2+, Fe2+, Fe3+ and Al3+ was mixed with the sulfate – GQDs 100 mg/L in final volume of 10 mL, the quenching effect of Hg2+ was strongly obtained, while other cations showed no change in their fluorescence intensities, except Al3+ gave slightly an enhancing effect compared with that of blank (sulfate – GQDs without cation) solution. In this case, fluorescence turn-on mode was preferred, and Al3+ could exhibit slightly the turn-on mode. So, optimization conditions for the sensitivity test of Al3+ concentration would be further investigated.
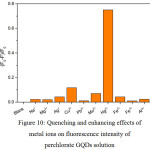 |
Figure 10: Quenching and enhancing effects of metal ions on fluorescence intensity of perchlorate GQDs solution Click here to View figure |
From Fig. 10, the fluorescence intensity of perchlorate decorated GQDs solution was drastically very less than that of undecorated GQDs solution. When 1.0 mL each of 10 µM of Na+, Mg2+, Ag+, Cu2+, Pb2+, Mn2+, Hg2+, Fe2+, Fe3+ and Al3+ was mixed with perchlorate – GQDs 100 mg/L in final volume of 10 mL, only strong quenching effect of Hg2+ was completely obtained but its sensitivity might be limited. However, the fluorescence turn-on mode of the perchlorate – GQDs was preferred. Notably, it could be useful sensor probe for other compounds.
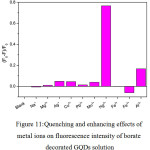 |
Figure 11: Quenching and enhancing effects of metal ions on fluorescence intensity of borate decorated GQDs solution Click here to View figure |
From Fig. 11, also the fluorescence intensity of borate decorated GQDs solution was drastically very less than that of undecorated GQDs solution, giving the same pattern as found in Fig. 9 and Fig. 10. When 1.0 mL each of 10 µM of Na+, Mg2+, Ag+, Cu2+, Pb2+, Mn2+, Hg2+, Fe2+, Fe3+ and Al3+ was mixed with the borate – GQDs 100 mg/L in final volume of 10 mL, the quenching effect of Hg2+ was strongly obtained. The second one was Al3+which gave slightly quenching one, while other cations showed no change in their fluorescence intensities compared with that of blank (borate – GQDs without cation) solution. Actually, fluorescence turn-on mode for this GQDs system was preferred, and Fe3+ could exhibit slightly the turn-on mode but it was quite low sensitivity. Optimization conditions for the sensitivity test of Fe3+ concentration would be further investigated.
Conclusion
The synthesis of graphene quantum dots (GQDs) by simple thermolysis of citric acid was successfully carried out. Fluorescence spectrum of the blue GQDs solution with lmax excitation of 365 nm was characterized. The fluorescence intensity at lmax emission of 460 nm was used to calibrate their uniformity. The GQDs in neutral-base solution gave high fluorescence intensity without any effect of ionic strength and masking agent tested. The effects of various anionic (1%, w/w) decorated with GQDs while thermolysis were also studied. From the results, nitrate, phosphate and chloride decorated GQDs exhibited an enhancement of the fluorescence intensity drastically, while perchlorate, borate and sulfate decorated GQDs gave their intrinsic quenching effects compared with that of bare GQDs. These anionic – decorated GQDs were subject for selectivity test with various metal ions. It was found that individual anionic decorated GDQs showed useful trends in their fluorescence turn-on/off, demonstrating that the as-prepared GQDs could be applied widely for further fluorescence sensors.
Acknoeledgements
The authors thank the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster of Khon Kaen University, Materials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry (PERCH-CIC), Thailand for financial support.
References
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Science 2008, 320, 356-358.
CrossRef - Baker, S.N.; Baker, G.A. Angew. Chem. Int. Ed. 2010, 49, 6726-6744.
CrossRef - Zhu, S.; Tang, S.; Zhang, J.; Yang, B. Chem. Commun.2012, 48, 4527-4539.
CrossRef - Li, L.L.; Wu, G.H.; Yang, G.H.; Peng, J.; Zhao, J.W.; Zhu, J.J. Nanoscale, 2013, 5, 4015-4039.
CrossRef - Shen, J.; Zhu, Y.; Yang, X.; Li, C.Chem. Commun. 2012, 48, 3686-3699.
CrossRef - Zhu, S.J.; Zhang, J.H.; Tang, S.J.; Qiao, C.Y.; Wang, L.; Wang, H.Y.; Liu, X.; Li, B.; Li, Y.F.; Yu, W.L.; Wang, X.F.; Sun, H.C.; Yang, B Adv. Funct. Mater.2012, 22, 4732-4740.
CrossRef - Liu R.; Yang, R.; Qu, C.; Mao, H.; Hu, Y.; Li, J.; Qu, L. Sens. Actuators. B. 2017, 241, 644-651.
CrossRef - Amjadi, M.; Manzoori, J.L.; Hallaj, T.J. Lumin.2014, 53, 73-78.
CrossRef - Liu, J.J.; Chen Z.T.; Tang, D.S.; Wang, Y.B.; Kang, L.T.; Yao, J.N. Sens. Actuators. B. 2015, 212, 214-219.
CrossRef - Zhou, C.; Jiang, W.; Via, B.K. Colloids Surf.B. 2014, 118,72-76.
CrossRef - Wu, Z.; Li, W.; Chen, J.; Yu,C. Talanta. 2014, 119, 538-543.
CrossRef - Hormozi-Nezhad, M.R.; Taghipoura, M. Anal. Methods. 2016, 8, 4064-4068
CrossRef - Kim, K.; Nam, Y.S.; Lee, Y.; Lee, K.B. J. Anal. Methods Chem. 2017,doi.org/10.1155/2017/3648564
CrossRef - Chen, L.; Wu,C.; Du, P.; Feng, X.; Wu, P.; Cai, C. Talanta. 2017, 164, 100-109.
CrossRef - Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Anal. Chem. 2014, 86, 10201-10207.
CrossRef - Tripathy, S.K.; Woo, J.Y.; Han, C.S. Sens. Actuators.B. 2013, 181, 114-118.
CrossRef - Chowdhury, A.D.; Doong, R.A. ACS Appl. Mater. Interfaces. 2016, 8, 21002-21010.
CrossRef - Xuan, W.; Ruiyi, L.; Saiying, F.; Zaijun, L.; Guangli, W.; Zhiguo, G.; Junkang, L. Sens. Actuators. B. 2017, 243, 211-220.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









