The Synthesis of 2,3-Dihydroquinazolin-4(1H)-Ones using Zinc Oxide Nanotubes Modified by Sio2 as A Catalyst with Recyclable Effect
Rakhshan Hakimelahi1,2 and Mohammad H. Mousazadeh3
and Mohammad H. Mousazadeh3
1Department of Chemistry, Jahrom Branch, Islamic Azad University, Jahrom, Iran.
2Department of Chemistry, Shiraz Branch, Islamic Azad University, Shiraz, Iran.
3Department of Chemistry, Amirkabir University of Technology, Tehran, Iran.
Corresponding Author E-mail: hakimelahi@jia.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/340109
zinc oxid nano tubes have attracted much attention in recent years. Hardly, direct uses of zinc oxid nano tubes which modified by SiO2 as a catalyst with recyclability has been applied for several organic reactions. The average particle size of ZnO catalysts is 57 nm, it has been characterized by XRD. Nano tubes surfaces have high density defects. The condensation of isatoic anhydride and an aromatic aldehyde exposed to ammonium acetate in the presence of few amounts of zinc oxid nano tubes modified by SiO2 as a catalyst can be a simple, suitable and efficient method for the quinazolin derivatives synthesis, the positive aspect is recyclable ability of the catalyst. It is clear that some characterization of catalyst such as combination of the small particle size and high-density surface defects of zinc oxid nano tubes which modified by SiO2 is the basic reason for unique catalytic activity of the catalyst. The practical and simple method led to unbeatable yields of the 2,3-Di hydro quinazolin-4(1H)-one derivatives at short times under mild conditions.
KEYWORDS:Reusable Catalyst; 2,3-Dihydroquinazolin-4(1H)-one Derivatives; Zinc Oxid Nanotubes; Sio2
Download this article as:| Copy the following to cite this article: Hakimelahi R, Mousazadeh M. H. The Synthesis of 2,3-Dihydroquinazolin-4(1H)-Ones using Zinc Oxide Nanotubes Modified by Sio2 as A Catalyst with Recyclable Effect. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Hakimelahi R, Mousazadeh M. H. The Synthesis of 2,3-Dihydroquinazolin-4(1H)-Ones using Zinc Oxide Nanotubes Modified by Sio2 as A Catalyst with Recyclable Effect. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42495 |
Introduction
Heterogenization of homogeneous catalysts has been a fantastic area of research from the industrial point of view. This approach, relieves the ability to use simultaneously the benefits of homogeneous catalysts (high activity and selectivity, etc.) and engineering promotes of heterogeneous catalysts (easy catalyst separation, easily regeneration, long catalytic life, thermal stability and recyclable ability) [1]. Since solid acids have many promotes such as decreased reactor and depletion of plant corrosion problems, simplicity in handling and more environmentally safe disposal [2–7], the application of solid acids have important role in organic transformation. 2,3-di hydro quinazolinones are a class of hetero cycles which possess a wide range of high activities, pharmaceutically including anti fertility, antibacterial, antitumor, anti-fungi and Monoamine-oxidase inhibitor (MAOI) [8–11], so they have attracted much attention recently. Several synthetic methods for the preparation of 2,3- dihydro quinazolinones have been reported, the most direct procedure includes accumulation of alkyl, aryl and heteroaryl aldehydes with anthranilamide in the presence of p-toluene sulfonic acid as a catalyst [12–14]. Also, the synthesis of 2,3-dihydroquinazolinones was accomplished by reductive cyclisation of o-nitro benzaldehyde or oazido benzamide [15,16]. Some of the stylish reported methods include materials as isatoic anhydride, and aldehydes exposed to ammonium acetate or primary amine in the presence of different types of reagents or catalysts, namely p-toluenesulfonic acid [17], amberlyst-15 microwave-assisted [18], montmorillonite K-10 [19], silica sulfuric acid [20], ionic liquid [21], Zn(PFO)2 [22], and ceric ammonium nitrate [23], silica-bonded N-propylsulfamic acid (SBNPSA) [24]. Recently, zinc oxid nanotubes are faced with increasing attentions because of fascinating chemical and physical characteristics and having potential technological applications [25], these are distinct both from those of the bulk phase and those from isolated atoms or molecules. The preparation [26] and applications [27,28] of nano-sized zinc oxid nanotubes modified by SiO2 have been already published in some papers and the reactions such as formylation and acetylation of hydroxyl groups have been reported [3].
Materials and Methods
Instrumentation
The commercial necessary materials were purchased from Fluka (Buchs, Switzerland), Merck (Darmstadt, Germany), and Aldrich(Germany). TLC (silica-gel 60 F254, n-hexane: ethyl acetate) was used for monitoring of the reaction. A FTIR Shimadzu- 470 Spectrometer was used for IR spectra. the 1HNMR Spectra were achieved with a Brucker-Instrument DPX- 400 MHz Avance 2 model. The similarity of their spectra and physical data were applied for identification of products and their characterizatons. The Powder X-ray diffraction (XRD) patterns were obtained on a Bruker Advance D8 Diffractometer with Cu Kα radiation (λ=0.154 nm). A Philips CM 200 FEG/HRTEM instrument (high resolution transmission electron microscope) operated at 200 Kv was usedd for TEM measurements.
Recyclability
One of the excellences of Zinc oxid nanotubes modified by SiO2 is separating them from the reaction mixture, on the other hand, they have recycling power. They participated as the same reaction again as a catalyst and their catalytic activity such as their efficacy was checked in subsequent reactions. Figure 1 shows the relation between the number of cycles of the reaction and the catalytic activity according to the yield.
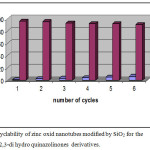 |
Figure 1: Recyclability of zinc oxid nanotubes modified by SiO2 for the formation of 2,3-di hydro quinazolinones derivatives. Click here to View figure |
General Procedure for the Synthesis of 2,3- dihydro quinazolinones
A mixture of isatoic anhydride (1 mmol), ammonium acetate (3 mmol) besides substituted aromatic aldehydes (1 mmol), zinc oxid nanotubes modified by SiO2 (1.0 mmol%) under solvent free conditions were applied at 120oCfor the appropriate time. In order to find when the reaction is completed, it should be monitored by TLC (thin layer chromatography). Finally, after completion, the reaction mixture should be cooled to the room temperature, filtered and washed with water (2 × 5 mL). Purification of the solid products were done by recrystallization of the catalyst from ethyl acetate, hence the catalyst was taken apart the pure product. Afterwards melting point of the product(s) were measured and the yeild of the reaction was determined.
Result and Discussion
The characterization of the catalyst was done by XRD; it shows high-density defects on nano tubes surfaces (Figure 2). Zinc oxid nano tubes modified by SiO2 as catalyst were used to establish the reaction of synthesis of derivatives of 2,3-Dihydro quinazolinones. The condensation reaction of isatoic anhydride and an aromatic aldehyde exposed to ammonium acetate ammonium acetate (1b) redounds to the product. To estimate the optimization conditions of the reaction, various amounts of zinc oxid nano tubes modified by SiO2 were taken in the range of 0.5-2.0 mmol% at 120oC (Table). The reaction surveys at different concentrations of the catalyst showed that the condensation reaction could be efficiently proceeded using 1 mmol% of the catalyst under solvent free conditions and thermal conditions.
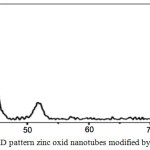 |
Figure 2: XRD pattern zinc oxid nanotubes modified by SiO2 catalyst. Click here to View figure |
Before, some studies have been showed new routes by using solid acid catalysts to synthesize heterocycles with favorable substitutes [29-32]. In this research has been reported a valid and an efficient procedure in order to synthesize 2,3-dihydroquinazolinones. This reaction is a kind of one-pot condensation which the reactants are isatoic anhydride, aldehydes and NH4OAC or primary amine. It has been performed in the presence of few amounts of zinc oxid nanotubes modified by SiO2 as an inexpensive catalyst (Figure 3). The effect of catalyst on the condensation of mentioned reactants was studied which was the corresponding of 2,3-dihydroquinazolinones production. The condensation reaction of isatoic anhydride, NH4OAc and 4-chloro-benzaldehyde was chosen as a model reaction in solvent free conditions (Table1). The results show clearly that zinc oxid nano tubes modified by SiO2 is an effective catalyst for this transformation whereas in the absence of zinc oxid nano tubes modified by SiO2 the reaction could not take place, even after 120 min (Table1, entry 1). The best result has been obtained with an amount of (1.0 mmol%) zinc oxid nano tubes modified by SiO2 in terms of reaction time and isolated yield. To demonstrate the generality of this process, many of aldehydes with divers substitution and different structurally were employed to synthesize the corresponding products which engendered very good yields (Table2). Also, a wide range of aniline derivatives and 2-phenylethyl amine were used and finally 2,3-dihydroquinazolinones were obtained in efficiency yield. The possibility of the catalyst recycling was examined, therefore the reaction of isatoic anhydride and 4- chloro benzaldehyde with NH4OAc in the presence of zinc oxid nano tubes modified by SiO2 was studied in solvent free conditions.
Table 1: The reaction of isatoic anhydride, ammonium acetate and 4-chlorobenzaldehyde exposed to different amounts of zinc oxid nano tubes modified by SiO2 catalyst in solvent freea conditions
|
Entry |
The amounts of catalyst (mmol) |
Time (min) |
Yield (%)b |
|
1 |
– |
120 |
– |
|
2 |
0. 5 |
30 |
84 |
|
3 |
1.0 |
20 |
92 |
|
4 |
2.0 |
20 |
92 |
aThe molar ratios of isatoic anhydride, NH4OAc and 4-chlorobenzaldehyde at 1200C have been found before.
bIsolated Yield.
The studies were extended with various aromatic aldehydes in order to prepare a series of 2,3-Di hydro quinazolinones derivatives, the generality of the reaction was demonstrated in optimized conditions (Scheme 1).
 |
Scheme 1: synthesis of 2,3-dihydroquinazolinones catalyzed with zinc oxid nano tubes modified by SiO2 in solvent free conditions. Click here to View figure |
Table 2: Synthesis of 2,3-dihydroquinazolinone derivatives catalyzed with zinc oxid nano tubes modified by SiO2 in solvent free conditions.
|
Entry |
R1 |
R2 |
Product |
Time (min) |
Yield (%)b |
|
1 |
4-CH3C6H4 |
H |
3a |
120 |
86 |
|
2 |
4-CH3OC6H4 |
H |
3b |
2.5 |
88 |
|
3 |
4-ClC6H4 |
H |
3c |
80,120c |
84, 81c |
|
4 |
4-BrC6H4 |
H |
3d |
3.0 |
87 |
|
5 |
2-Thionyl |
H |
3e |
3.0 |
81 |
|
6 |
4-CH3C6H4 |
C6H5 |
3f |
3.0 |
84 |
|
7 |
4-ClC6H4 |
C6H5 |
3g |
2.5 |
86 |
|
8 |
4-FC6H4 |
C6H5 |
3h |
3.0 |
83 |
|
9 |
2-Thionyl |
C6H5 |
3i |
3.0 |
83 |
|
10 |
4-CH3C6H4 |
C6H5CH2CH2 |
3j |
3.0 |
83 |
|
11 |
4-CH3C6H4 |
4-HO-C6H4 |
3k |
3.0 |
82 |
aThe molar ratios of isatoic anhydride: aldehyde: ammonium acetate has been found before.
(1 mmol%) zinc oxid nano tubes modified by SiO2, at solvent free condition.
bIsolated Yield.
cThe reaction was accomplished exposed to reused catalyst.
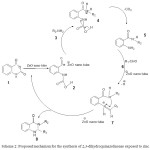 |
Scheme 2: Proposed mechanism for the synthesis of 2,3-dihydroquinazolinones exposed to zinc oxid nano tubes modified by SiO2. Click here to View figure |
As it was pointed, the reaction mixture was solvent free. The product was recrystallized and separated from catalyst using hot ethanol. The catalyst after recycling could be employed four times without any treatment. Catalyst turnover is perfect and there is no decreased efficiency(Table2). For the mentioned one-pot reaction, some possible mechanisms had been reported before by Baghbanzadeh, co-workers [17] and Wang et al. [22]. According to the experimental results, a credible mechanism has been proposed in Scheme 2. In the presence of zinc oxid nano tubes which is modified by SiO2 as catalyst, first the isatoic anhydride (1) is rearranged and formed an intermediate (2) with positive charge on carbonyl group, then the N-nucleophilic amine (3) attack occurs on the carbonyl group of the intermediate (2) to produce another species as intermediate (4), which after decarboxylation reaction followed by proton transfer, in turn affords the intermediate named (5). Subsequently, the aldehyde activated by zinc oxid nanotubes modified by SiO2, participate in a condensation reaction with (5) to proceeds the imine intermediate (7), which the general cyclic process forms finally yields proposed product (8). In conclusion, heterogeneous conditions get easy and clean work-up approach, with high yields which this method could be practical for multi-component reactions because of the catalyst recovery. So the present methodology could be a significant manner which adds to other methods.
Compound 3i: mp 198–200 8C. IR (neat): 3285.62, 3024.37, 1631.68, 1609.55, 1503.79, 1483.88, 1387.95, 1366.74, 1317.89, 1258.12, 1191.04, 1158.03, 1069.94, 836.88, 793.58, 754.82, 713.91, 694.77 cm-1. 1H NMR (400 MHz, DMSO-d6): d 6.53 (d, 1H, J = 2.5 Hz), 6.77 (dt, 1H, J1 = 7.55 Hz, J2 = 1.01 Hz), 6.82 (d, 1H, J = 7.81 Hz), 6.86 (dd, 1H, J1 = 5.03 Hz, J2 = 3.51 Hz), 6.97 (d, 1H, J = 3.52 Hz), 7.21-7.26 (m, 1H), 7.32-7.41 (m, 6H), 7.72 (d, 1H, J = 2.75 Hz), 7.73 (dd, 1H, J1 = 7.81 Hz, J2 = 1.5 Hz). 13C NMR (100 MHz, DMSO-d6): d 69.56, 115.28, 115.66, 118.15, 125.93, 126.34, 126.36, 126.40, 126.52, 127.97, 128.71, 133.84, 140.49, 144.63, 146.38, 161.59. Anal. Calcd for C18H14N2OS: C, 70.62; H, 4.63; N, 9.16; S, 10.51; Found: C, 70.41; H, 4.52; N, 9.07; S, 10.36. Compound 3j: mp 152-154 8C. IR (neat): 3282.12, 3027.52, 1628.2, 1609.22, 1583.63, 1506.14, 1485.19, 1408.12, 1311.41, 1173.38, 822.38, 750.44, 693.89 cm-1. 1H NMR (400 MHz, DMSO-d6): d 6.31 (d, 1H, J = 2.5 Hz), 6.73(dt, 1H, J1 = 7.55 Hz, J2 = 1.01 Hz), 6.77 (d, 1H, J = 7.79 Hz), 7.11-7.23 (m, 3H), 7.28-7.33 (m, 3H), 7.32-7.37 (m, 2H), 7.41-7.46 (m, 2H), 7.65 (d, 1H, J = 2.5 Hz), 7.76 (dd, 1H, J1 = 7.83 Hz, J2 = 1.5 Hz). 13C NMR (100 MHz, DMSO-d6): d 72.09, 114.88, 115.18, 115.29, 115.34, 117.66, 126.14, 126.48, 127.99, 128.67, 128.79, 128.89, 133.85, 136.88, 140.64, 146.59, 160.69, 162.28, 163.11. Anal. Calcd for C23H22N2O: C, 80.69; H, 6.51; N, 8.22; Found: C, 80.53; H, 6.37; N, 8.03. Compound 3k: mp 249-253 8C. IR (neat): 3311.08, 3119.81, 3015.72, 2891.34, 2359.39, 1633.77, 1612.59, 1595.97, 1580.45, 1514.48, 1444.67, 1421.48, 1266.71, 1225.65, 1150.56, 1117.88, 1018.55, 870.02, 839.52, 806.59, 770.74, 752.55, 695.39cm-1. 1H NMR (400 MHz, DMSO-d6): d 2.11 (s, 3H), 5.94 (d, 1H, J = 2.5 Hz), 6.54-6.62 (m, 4H), 6.92 (dt, 2H, J1 = 8.83 Hz, J2 = 2.02 Hz), 6.99 (d, 2H, J = 8.07 Hz), 7.12-7.15 (m, 3H), 7.39 (d, 1H, J = 2.28 Hz), 7.61 (dd, 1H, J1 = 7.64 Hz, J2 = 1.5 Hz), 9.35 (s, 1H). 13C NMR (100 MHz, DMSO-d6): d 20.73, 73.15, 114.62, 115.12, 115.42, 117.32, 126.56, 127.91, 127.95, 128.89, 132.24, 133.53, 137.60, 138.02, 146.64, 155.55, 162.34. Anal. Calcd for C21H18N2O2: C, 76.44; H, 5.51; N, 8.49; Found: C, 76.25; H, 5.33; N, 8.29.
Conclusion
In summary, we have established a new application of zinc oxid nanotubes modified by SiO2 as an effective, suitable and reusable catalyst. A practical and simple method which redounds to excellent yields of the 2,3-Di hydro quinazolin-4(1H)-one derivatives under mild conditions and within short times. The reason proposed for higher catalytic activity of zinc oxid nano tubes modified by SiO2 is a combination effect of the small particle size and high-density surface defects. In these reactions, ammonium acetate and different types of aromatic aldehydes as initial materials have potential green chemistry advantages. This method is reasonable from economic point of view and environmentally, due to little waste production. Some advantages like as simple preparation, stability of catalyst, easy handling, short reaction times, having a procedure with simple work-up and the high yields of products, put this reaction at the best existing methodologies. No necessary of using toxic organic solvents as media, is another positive sight.
Acknowledgement
The authors gratefully appreciate partial support of this research by the Islamic Azad University, Jahrom and Shiraz Branch, Iran.
References
- Choudhary, D; Paul, S; Gupta. R; Clark. J. H, Green Chem. 2006, 8 ,479-482.
CrossRef - Niknam, K; Saberi, D, Appl. Catal. 2009, 366, 220-225.
CrossRef - Niknam, K; Saberi, D, Tetrahedron Lett. 2009, 50, 5210-5214.
CrossRef - Karimi, B; Ghoreishi-Nezhad, M, J. Mol. Catal. A. Chem. 2007, 277, 262-265.
CrossRef - Melero, J. A.; Van Grieken, R; Morales, G, Chem. Rev. 2006,106, 3790-3812.
CrossRef - Niknam, K; Saberi, D; Nouri Sefat, M, Tetrahedron Lett. 2009, 50, 4058-4062.
CrossRef - Niknam, K; Saberi, D; Molaee, H; Zolfigol, M. A. Can. J. Chem. 2010, 88, 164-171.
CrossRef - Sadanadam, Y. S.; Reddy, R. M.; Bhaskar, A. Eur. J. Med. Chem. 1987, 22, 169-173.
CrossRef - Yale, H. L.; Kalkstein, M. J. Med. Chem. 1967, 10, 334-336;
CrossRef - Bonola, G.; Sianesi, E. J. Med. Chem. 1970, 13, 329-332.
CrossRef - Hour, M.; Huang, L.; Kuo, S. J. Med. Chem. 2000, 43, 4479-4487.
CrossRef - Gupta, R. C.; Nath, R.; Shanker, K. J. Indian Chem. Soc. 1979, 56, 219-220; (b) Davoodnia, A.; Bakavoli, M.; Khorramdelan, F. Indian J. Heterocycl. Chem. 2006, 16, 147-150.
- Sharma, S. D.; Kaur, V. Synthesis, 1989, 677-680.
CrossRef - Moore, J. A.; Sutherland, G. J.; Showery, R. J. Org. Chem. 1969, 34, 887-892.
CrossRef - Qiao, R. Z.; Xu, B. L.; Wang, Y. H. Chin. Chem. Lett., 2007, 18, 656-658.
CrossRef - Su, W.; Yang, B. Aust. J. Chem. 2002, 55, 695-697.
CrossRef - Shi, D.; Rong, L.; Wang, J.; Zhuang Q.; Wang, X.; Hu, H. Tetrahedron Lett. 2003, 3199-3201.
CrossRef - Baghbanzadeh, M.; Salehi, P.; Dabiri, M.; Kozehgary, G. Synthesis, 2006, 344-348.
- Surpur, M. P.; Singh, P. R.; Patil, S. B.; Samant, S. D. Synth. Commun. 2007, 37, 1965-1970.
CrossRef - Salehi, P; Dabiri, M.; Baghbanzadeh, M.; Bahramnejad, M. Synth. Commun. 2006, 36 2287-2292.
CrossRef - Dabiri, M.; Salehi, P.; Baghbanzadeh, M. Catal. Commun. 2008, 9, 785-788; (b) Salehi, P.; Dabiri, M.; Zolfigol, M. A. Tetrahedron Lett. 2005, 46, 7051-7053.
- Dabiri, M.; Salehi, P.; Baghbanzadeh, M. Monatsh. Chem. 2007, 138, 1191-1194.
CrossRef - Wang, L. M.; Hu, L.; Shao, J. H.; J. Fluorine Chem. 2008, 129, 1139-1145.
CrossRef - Baghbanzadeh, M.; Dabiri, M.; Salehi, P. Heterocycles, 2008, 75, 2809-2815.
CrossRef - Niknam, K.; Jafarpour, N.; Niknam, E. Chinese Chemical Letters, 2011, 22, 69-72.
CrossRef - Pandit, S. H.; Bhalerao, S. W. J.Chem.Sci , 2011, 123 , 421-426.
CrossRef - Nagalakshmi, G. E. J. Chem. 2008, 5, 447-452.
- Shivaji, P.; Swapnil, B. j. Chem. Sci, 2011, 123, 421-426.
- Mombini Godazhdar, B.; Niknam, B., J. Iranian Chem. Res, 2012, 5 (4), 231-239.
- Niknam, K.; Sabari, D.; Mohagheghnejad, M. Molecules, 2009, 14, 1915-1926.
CrossRef - Niknam, K.; Saberi, D.; Baghernrjad, M. Chin. Chem. Lett. 2009, 20, 1444-1448.
CrossRef - Niknam, K.; Saberi, D.; Sadegheyan, M.; Deris, A. Tetrahedron Lett. 2010, 51, 692-694.
CrossRef - Niknam, K.: Panahi, F.; Sabari, D.; Mohagheghnejad, M. J. Heterocycl. Chem. 2010, 47 292-300.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









