The Preparation of All-Cellulose Nanocomposite Film from Isolated Cellulose of Corncobs as Food Packaging
M. Zulham Efendi Sinaga1, Saharman Gea1 , Nami Panindia1 and Yuan Alfinsyah Sihombing2
, Nami Panindia1 and Yuan Alfinsyah Sihombing2
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Sumatera Utara, Medan, 20155, Indonesia.
2Department of Physics, Faculty of Mathematics and Natural Sciences, University of Sumatera Utara, Medan, 20155, Indonesia.
Corresponding Author E-mail: s.gea@usu.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340166
The all-cellulose nanocomposite as food packaging has been prepared from the isolated cellulose of corncobs waste. The cellulose in corncob powder was isolated through a standard isolation method with various acid and bases by the heating process. As many as 17.4 % of cellulose was successfully isolated and characterized via FTIR spectroscopy, TGA and TEM. Furthermore, the isolated cellulose was partially dissolved in DMAC/ LiCl 8 % and compressed at high temperature to produce the all-cellulose nanocomposite film. The isolated cellulose showed a similar functional group with the commercial cellulose, indicating the success of the isolation process. SEM analysis was conducted to several all-cellulose nanocomposites with various time of soaking. The best soaking time to perform was 60 minutes, showing a good result in morphological surface analysis. Moreover, the all-cellulose nanocomposite film exhibited as a good food packaging material to negate the microbes growth in food.
KEYWORDS:Cellulose; All-Cellulose Nanocomposite Film; Food Packaging; Packaging Bread
Download this article as:| Copy the following to cite this article: Sinaga M. Z. E, Gea S, Panindia N, Sihombing Y. A. The Preparation of All-Cellulose Nanocomposite Film from Isolated Cellulose of Corncobs as Food Packaging. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Sinaga M. Z. E, Gea S, Panindia N, Sihombing Y. A. The Preparation of All-Cellulose Nanocomposite Film from Isolated Cellulose of Corncobs as Food Packaging. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41517 |
Introduction
The use of plastic packaging has dominated the food industry in Indonesia, replacing the use of glass and metal packaging. The use of plastics as food packaging materials are mainly caused by its superiority in terms of a flexible form; made it easy to follow the shape of the food, easy to be labeled, not easily broken, and can be massively produced. Meanwhile, the price is affordable, and there is a wide selection of plastic materials. However, the use of plastic as packaging material has raised various environmental problems, such as; unrecyclable, cannot be naturally decompossed by microbes in the soil, the accumulation of plastic waste that causes pollution and damage to the environment. Another weakness is the main ingredient of plastic that derived from petroleum, whichits resourcesare dwindling and can not be renewed.Studies have shown that the use of biopolymer-based materials may minimize the packaging waste production. Moreover, it consecutively solves the waste disposal problem and to some extent owing to its biodegradability. It is based on research that have been conducted by using materials from nature to produce an environmentallyfriendly packaging material.Comprehensive research has been done to develop the alternative of packaging materials by decreasing the environmental impact of the petroleum based on packaging materials.1
Corn is one of the agricultural products that are widely produced in Indonesia, particularly in North Sumatra. Data showed that the development of corn agriculture in North Sumatra, according to the Central Bureau of statistics in 2015 was 1,519,407 tons. Moreover, based on the data generated, the corncob waste was as much as 455,822 tons in 2015. Several studies have been reported to utilize the corn cobswaste as bioethanol feed livestock. Cellulose is the most abundant natural biopolymer and is a linear homo-polysaccharide composed of β-D-glucopyranose units connected by β-1-4-linkages with a repeating unit of cellobiose2. Cellulose consists of both crystalline and amorphous domains. There are three hydroxyl groups in a monomer of the cellulose structure that form hydrogen bonds, which possesses a vital role in the physical properties and crystalline packing of cellulose3. The high content of cellulose in corn cobs can be used as a source of cellulose which can then be used for making the all-cellulose nanocomposite film that serves as a food packaging material and is environmentally friendly as it can be degradable in nature.
Composite is a mixture of two or more substances which generally has different properties, so that the final results will produce a material which has new or unique properties result of the combination of material properties which are mixed. Currently, there is a development of a single composite such as the composite cellulose (all-cellulose composite), where the matrix is only cellulose.4–5However, as the development of technology nowadays has increased significantly, a single composite that has been chopped as nanoparticles has been produced.
Material and Methods
Materials
The materials used in the research were corn cobs, NaOH pellets, NaOCl(c) 12%, H2O2(c)30%, NaNO2, Na2SO3, HNO3(c) 65%, Dimethyl acetamide (DMAC), and LiCl. These materials were purchased from Merck.
Methods
Isolation of Cellulose from Corn Cob
A total of 75 grams of powdered corn cobs was added in a beaker glass, then 1 litre of HNO3(3.5%) and 1 mg of NaNO2 were added and heated at 90°C for 2 hours with constant stirring on a hot plate. Then, the mixture was filtered, and washed until the filtrate residue reached the neutral pH. The filtrate was then added with 375 mLof NaOH(2%) and heated for 1 hour at 50°C with constant stirring on a hot plate, then, it was filtered and washed until the filtrate residue reached the neutral pH. The filtrate was then added with 500 mLof NaOCl(1.75 %) and heated at 70°C for 30 minutes with constant stirring on a hot plate, filtered and washed until the filtrate residue reached the neutral pH. The filtrate was then added with 500 mL NaOH(17.5 %) and heated at 80°C for 30 minutes with constant stirring on a hot plate, then it was filtered and washed until the filtrate residue reached the neutral pH. The filtrate was then added with 250 mL of H2O2(10%) and heated at 60°C for 15 minutes with constant stirring on a hot plate, then, it was filtered and washed until the filtrate residue reached the neutral pH. Furthermore, the filtrate was dried at 60°C (cellulose) in the oven, and stored in a desiccator.6The cellulose obtained were characterized by using FTIR, TGA, and TEM
Preparation of All-Cellulose Nano Composite Film
The cellulose was activated by immersion process in distilled water, acetone, and N, N- dimethylacetamide (DMAc) respectively, for 1 hour at room temperature for each solvent. After that, the activated cellulose was dissolved in DMAc/LiCl(8%) (ratio 1:10) solvent with the variations of dissolution time in 0, 30, 60, 90, 120, and 150 minutes at room temperature. Moreover, the all-cellulose was dried in the oven at 80°C and then pressed at a high temperature of 150°C for 1 hour to prepare all-cellulose nanocomposite film. Then, all-cellulose nanocomposite film was tested for their thermal properties by TGA, surface analysis using SEM, crystallinity test by XRD, tensile strength and total number plates test for the application of the film.
Results and Discussion
Isolated Cellulose from Corn Cob and Preparation of All-Cellulose Nanocomposite Film
After a delignification, bleaching, and purification of corn cobs have been conducted, the white cellulose powder was produced. For 75 grams of corn cobs powder used, it would produce 13.03 grams of cellulose (17.4% of the initial mass of corncobs powder). The cellulose produced was subsequently and partially dissolved in DMAC/LiCl 8% (1:10), which wiould give a gel and then compressed at hot temperature to produce all-cellulose nanocomposite film.
The Functional Groups Analysis using FT-IR Spectroscopy
The functional groups of cellulose which was isolated from corn cobs with commercial cellulose shown the same pattern. It indicated that the isolation of cellulose from corn cobs was successful. The results of FTIR analysis of each sample can be seen in Figure 1 and Table 1.
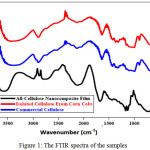 |
Figure 1: The FTIR spectra of the samples Click here to View figure |
Table 1: The functional groups recorded from the samples
| Functional groups | Isolated cellulose from corn cobs (cm-1) | Commercial cellulose (cm-1) | All-cellulose nanocomposite film (cm-1) |
| Stretching vibration O-H | 3406.29 | 3367.71 | 3560.59 |
| Stretching vibration C-H | 2897.08 | 2897.08 | 2877.79 |
| Stretching vibration C-O-C | 1149.57 | 1099.43 | 1130.29 |
The presence of acetyl ester groups on hemicellulose or carboxyl groups on ferulic acid and p-coumaric on lignin, are represented in the absorption wavelength around 1700 cm-1 which confirms the C = O group7. According to the Figure 1, the absorption wavelength around 1700 cm-1 was not visible, so it can be concluded that the content of nanocellulose had been lost during the treatment process. Moreover, the corn cob cellulose insulation has been successfully carried out. For the all-cellulose nanocomposite films, it produced the same functional groups band recorded with cellulose, but there was a movement of wavenumber at this area due to the dilution effect of DMAC/ LiCl as a solvent.
TEM
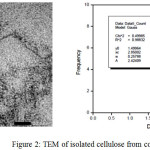 |
Figure 2: TEM of isolated cellulose from corn cobs Click here to View figure |
XRD
Based on the results of XRD analysis (Figure 3), the isolated cellulose and all-cellulose nanocomposite films at 0 minute were still having crystal in their compositions.
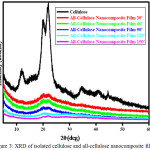 |
Figure 3: XRD of isolated cellulose and all-cellulose nano composite films Click here to View figure |
However, the longer the dissolution time, the crystallinity properties tend to decrease. The dissolution process will result in a decrease in the size of the crystals and this happened because of the solvent can penetrate the space between the amorphous and crystalline so that the chain will be dissolved5,8,9.
The TGA test Results
Based on the results of TGA (Fig 4), it is noticed that the starting point of the isolated cellulose decomposition was 331oC while all-Cellulose nanocomposite was 238oC. The highlight of the isolated cellulose degradation was at 368oC while all-Cellulose nanocomposite was at 255oC. Residues of the isolated cellulose were 9.75% while the all-cellulose nanocomposite was 25.38%. In average, it showed that the thermal stability of the all-cellulose nanocomposite was lower than the cellulose. This was due to the effect of dissolving the cellulose in LiCl/ DMAC and the degree of crystallinity of all-cellulose nanocomposite that was lower than the cellulose.
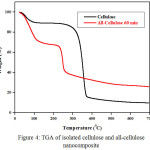 |
Figure 4: TGA of isolated cellulose and all-cellulose nano composite Click here to View figure |
Tensile Strength
Mechanical properties are one of the important properties of a composite and also in the manufacture of a food packaging. Based on the tensile strength results (Fig 5), it can be concluded that the best mechanical properties were owned by a sheet of all-cellulose nanocomposite which were soaked for 60 minutes in LiCl/ DMAC (8%) with the values of tensile strength of 2.254 MPa, elongation at break 21.55% and modulus of elasticity of 0.1045 respectively.
 |
Figure 5: The tensile strength test results Click here to View figure |
SEM Analysis
To determine the surface of all-cellulose composite produced, SEM analysis has been conducted. Based on Fig 6, it can be seen that the surface of all cellulose nanocomposite which was dissolved partially in LiCl/ DMAC (8%) for 60 minutes has a more tidy surface compared to the surface of all cellulose nanocomposite with different time of dissolution. It might be due to the longer the dissolution process, which made the surface of all-cellulose more soluble. The tidier surface of the all-cellulose nanocomposite also affects the value of the tensile strength of all-cellulose nanocomposite.
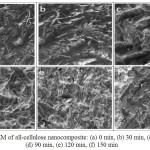 |
Figure 6: SEM of all-cellulose nanocomposite: (a) 0 min, (b) 30 min, (c) 60 min, (d) 90 min, (e) 120 min, (f) 150 min Click here to View figure |
Total Plate Count Test
The all-cellulose nanocomposite application to the food was needed to know if the resultant composite may block or reduce the damage to the food from external factor such as microbes that are not profitable. In this study, the all-cellulose nanocomposite was applied to wrap the bread for several days and the results have been shown in Fig. 7.
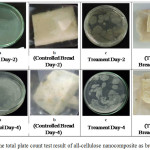 |
Figure 7: The total plate count test result of all-cellulose nanocomposite as bread wrapping Click here to View figure |
Acknowledgement
The authors would like to send a gratitude to the Rector of University of Sumatera Utara for the financial support towards this research in the TALENTA Project 2016
Conclusion
From the research that has been done, it can be concluded that the cellulose from corn cobs have been isolated as evidence by FTIR, wherein the functional group of cellulose isolated from corn cobs in accordance with commercial cellulose. All-cellulose produced nanocomposite which has the potential as food packaging materials.
References
- Rhim, J. W.; Ng, P. K. W. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433
CrossRef - Abdul Khalil, H. P.; Davoudpour, Y.; Islam, M. N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Carbohydrate Polymers, 2014, 99, 649–665
CrossRef - Gea, S.; Bilotti, E.; Reynolds, C. T.; Soykeabkeaw, N.; Peijs, T. Mater. Lett. 2010, 64(8), 901–904
CrossRef - Huber, T.; Pang, S.; Staiger, M. Composites Part A: Applied Science and Manufacturing, 2012, 43(10), 1738–1745
CrossRef - Nishino, T.; Matsuda, I.; Hirao, K. Macromolecules, 2004, 37(20), 7683–7687
CrossRef - Ohwoavworhua, F. O.; Adelakun, T. A. Tropical Journal of Pharmaceutical Research. 2005, 4(2), 509-516
- Alemdar, A.; Sain, M. Bioresour. Technol. 2008, 99(6), 1664–1671
CrossRef - Duchemin, B. J. C.; Mathew, A. P.; Oksman, K. Compos. Part A Appl. Sci. Manuf. 2009, 40(12), 2031–2037
CrossRef - Yousefi, H.; Nishino, T.; Faezipour, M.; Ebrahimi, G.; Shakeri, A.; Morimune, S. Adv. Compos. Lett. 2010, 19(6), 190–195

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









