Synthesis of Mesoporous Fe2O3/SBA-15 and its Application for Nickel (II) Ion Adsorption
Maria Ulfa and Arini Miftahul Jannah
and Arini Miftahul Jannah
Chemistry Education, Faculty of Teacher Training and Education, Sebelas Maret University, Jl. Ir. Sutami 36A Surakarta, Central Java-57126, Indonesia.
Corresponding Author E-mail: ulfa.maria2015@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340145
The uniform mesoporous Fe2O3/SBA-15 has been succesfully synthesized by classical impregnation of iron nitrate acid in the presence of mesoporous silica SBA-15 followed by ultrasonication, microwave treatment and calcination. The characterization of mesoporous Fe2O3/SBA-15 material structures using FTIR spectroscopy , powder X-Ray Diffraction, Nitrogen adsorption-desorption, Transmission Electron Microscopy, and Energy Dispersive X-Ray. The result of FTIR analysis showed that mesoporous Fe2O3/SBA-15 silica material sieves had silanol (Si-OH), siloxyl (Si-O-Si), and Fe-O, while the XRD analysis showed that iron oxide was completely impregnated on mesoporous silica SBA-15 surface. Nitrogen adsorption-desorption analysis showed that surface area of mesoporous Fe2O3/SBA-15 material was 479 m2/g with volume of 0.87 cm3/ g, and a pore diameter of 6.50 nm. Adsorption Performance of Fe2O3/SBA-15 material investigated by using nickel (II) ions as adsorbate model. The optimum contact time result was analyzed with adsorption kinetics model. The optimum contact time of adsorption of Fe2O3/SBA-15 mesoporous material to Ni (II) ion was 240 minutes with optimum adsorption capacity of 352.982 mg / g. The optimum contact time data shows that the Fe2O3/SBA-15 silica mesoporous material in adsorption of nickel (II) ions follows the Lagergren kinetic model.
KEYWORDS:Mesoporous Silica SBA-15; Iron Oxide; Wet Impregnation; Ultrasonic; Microwave; Adsorption; Niickel Ions
Download this article as:| Copy the following to cite this article: Ulfa M, Jannah A. M. Synthesis of Mesoporous Fe2O3/SBA-15 and its Application for Nickel (II) Ion Adsorption. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Ulfa M, Jannah A. M. Synthesis of Mesoporous Fe2O3/SBA-15 and its Application for Nickel (II) Ion Adsorption. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=43011 |
Introduction
Nickel is one of the toxic metals cause water contamination. The source of nickel pollution is industrial processes such as electroplating, galvanization, plastics manufacturing, metal finishing, batteries manufacturing, mining, and dyeing operations [1,2]. The normal level of nickel concentration that permissible in water enviroment is at range 0.05-0.12 mg/m3. The concentration that higher than normal level causing kidney, headaches, stomagaches, hepatitis as several problem of health. Conventional methods for the removal Ni (II) from wastewaters has been widely performed by researchers include ion-exchange[3], electrodialysis and electrocoagulation [4], floatation [5], coagulation and flocculation [6], membrane technology [7], biofiltration method [8] and adsorption [9]. In general, the high value of method evaluated by a number of requirement such as remove major and minor wastes, low cost, simple and green for environment. From several method, the favourable method for minimize metal ion in water is adsorption. Alot of previous report using adsorption method succes to remove metal ion of cadmium, mercury, iron, barium and toxic molecul of fenol, tiophene, naphtalene and dibenzotiophene
In special case for removing nickel ion, there are many of adsorbent using to minimize this metal such as activated carbon, silica, agricultural, microbes, and chitin [10-15]. The new economic material with not only high sustainability but also has a high adsorption capacity urgently to develope in this decade as a solution for the enviromental problem. Currently, mesoporous silica materials attract attention by researchers because it has many advantages such as a high thermel stability, uniform structure, large diameter, high specific surface area and rich with silanol on the framework [11-17]. The most unique of mesoporous silica is mesoporous silica SBA-15 due to the honeycomb shape structure with diameter size up to 15 nm, pore volume up to 1,5 cm3/g and specific surface area up to 2500 m2/g [12-19]. The mesoporous SBA-15 material silica sieves can be applied in various fields such as adsorption, separation, and catalysis [14-22]. In the adsorption/desorption process, controlling process of the mesoporous silica SBA-15 material showed good thermal stability, physical stability and sructural homogeneity [15-23]. In the other side, the mesoporous silica SBA-15 have problem in separation process. When the mesoporous silica SBA-15 have been adsorbed metal ion, it need special treatment to recover SBA-15 from adsorbate. One of favourable separation methode is using magnetic component due to the efficiency, not time consuming and economic reason.In the best of our knowledge, the investigation of SBA-15 material in separation section after adsorption process using magnetic element is rarely done.
In our present work, we try to add magnetic element in the mesoporous silica SBA-15. The modification on mesoporous wall-surface was carried out by doping the iron with the magnetic characteristic. We choose iron as magnetic element to dispersed into the mesoporous silica SBA-15 due to the available stock reason and high magnetic character on it. The objective of this research was to synthesize of adsorbent mesoporous silica SBA-15 (Fe2O3/SBA-15) modified with iron as metal doping for Ni (II) adsorption using collaboration methods such as wet impregnation, ultrasonic, and microwave. The characteristic of materials was analyzed using FTIR, XRD, BET, TEM, and EDX. We hope can get some information about character of the mesoporous silica SBA-15 modifide by iron metal for future magnetic separation material.
Experimental
Materials
SBA-15 (pore diameter 7-9 nm, surface area 560 m2/g, pore size 8 nm, pore volume 1.0 cm3/g and the pore morphology is hexagonal) merk Six C Materia (China supplier), Fe(NO3)3.9H2O (Merck, pro analysis), HNO3 (Merck, 65%), HCl (Merck, 37%), and standard solution of Ni (II) (Merck, 1000 ppm).
Preparation of Fe2O3/SBA-15
The activation process of SBA-15 was performed by adding 1.5 g of SBA-15 into the 1 M HCl solution followed by stirring for 24 h in100 rpm in room temperature. The white slurry filtering with Whatman paper, washing with deionization water and drying at 100 ºC for 24 h. The wet impregnation process was preformed by mixxing a fix amount of Fe(NO3)3.9H2O solution as the precursor of the iron ion and 1 g of the activated SBA-15 to obtain 2 wt % Fe on the prepared SBA-15. The material was dispersed by ultrasonication for 1 h and microwave for 30 minutes with high temperature labeled Fe2O3/SBA-15.
Characterization of Fe2O3/SBA-15
FTIR spectra of Fe(NO3)3, SBA-15, and Fe2O3/SBA-15 were recorded on Shimadzu Prestige-21. XRD analysis of SBA-15 and Fe2O3/SBA-15 was performed using Rigaku Multiflex 0,64 kW. The samples were scanned at a 2θ diffraction angle which small angle starts from 2-5 ˚ and a wide angle starts from 10-80˚. The BET SBA-15 and Fe2O3/ SBA-15 such as surface area, volume, and pore diameter were analyzed using Quantacrome Nova 1200. For morphology of Fe2O3/SBA-15 was observed under transmission electron microscope. For the elements contained in the samples were calculated using EDX TSL Amatex with detector type: Sdd Apollo X dan resolution:127.89.
Adsorption of Ni (II) ions on Fe2O3/SBA-15
Adsorption experiments have been done with the batch mode in the sample bottle. The standard solution prepared with diluted 1000 ppm solution of Ni (II) 0.05 mol HNO3 in L-1. The 49 ml solution of Ni (II) contacted with 0,02 g adsorbent Fe2O3/SBA-15 and subsequently stirred at room temperature. The experiment was conducted on different adsorption times that is 5-840 minutes. Every sample was taken each of 4 ml of 59 ml solution of Ni (II) using a syringe and filtered through Whatman paper. Finally, the obtained filtrate was measured using AAS (Shimadzu AA 6650). The amount of Ni (II) adsorbed at time t by Fe2O3/SBA-15 using to obtain adsorption capacity. Its investigated by balancing mass calculation.

The initial and final concentration of nickel ion at time represented by Co and Ce (mg.L-1). The volume of nickel ion at time (ml) represented by V and the adsorption capacity of nickel ion represented by q
Result and Discussion
Fig 2 show the IR spectra of Fe2O3/SBA-15 as adsorbent. The adsorption band at 676,08 cm-1 and 1384 cm-1 was attributed to the iron oxide and nitrate ion (Fig. 2a). This is means that material in Fe2O3/SBA-15 containing some iron oxide as loading effect and the less number of nitrate ion that can not remove during calsination. The vibration adsorption peak of the silanol Si-OH bond, which was originally located at 1639,56 cm-1and siloxane Si-O-Si bond at 1085 cm-1 (Fig. 2b). Figure 2c, shows the absorption peak of the Si-OH band at 1635.71 cm-1 and Si-O-Si band at 1084.04 cm-1.The peak of slanol and siloxane sugested that the framework of mesoporous silica SBA-15 was quite stable after all step preparation process. However, the vibration peak of the Fe-O band at 600 cm-1is disappeared which indicated the iron-doped within SBA-15 in low number. All the FTIR result not different compare with IR spectra of mesoporous silica SBA-15 and iron in previous report [20]. This phenomenon means that iron modified in the surface of mesoporous silica SBA-15 has been succesfull synthesis in this work.
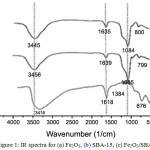 |
Figure 1: IR spectra for (a) Fe2O3, (b) SBA-15, (c) Fe2O3/SBA-15 Click here to View figure |
Diffraction patterns for Fe2O3, SBA-15, dan Fe2O3/SBA-15 are shown in Fig 2. In Fig 2 show that SBA-15 showed two typical diffraction peaks on the reflection(0 1 2)and (1 1 0) at 2θ 25.5° and 33.5°. The Small angle diffraction pattern (not shown) describe a typical mesoporous peak by samples at 2θ = 1.05°. The sample assynthesis Fe2O3/SBA-15 shows three reflection peaks (1 0 0), (110) and (2 2 0), this peaks has the similar mesoporous hexagonal structure as SBA-15. Fe2O3/SBA-15 contained prominent peaks at 1.1° and other peaks at 0.8° and 1.7°. Those peaks were the typical characterization of the hexagonal three-dimensional form indicated iron oxide doping completely within mesoporous channels of SBA-15 after impregnation treatment, ultrasonic and microwave. The ordered structure of the parent SBA-15 silica was changed after modification process. However, the diffraction peak intensity of the mesoporous silica SBA-15 decreased after iron added due to the iron act as strong X-ray absorber [22]. The typical peak of iron on XRD indicate that the presence of Hematite (Fe2O3) reinforced with spread elements of EDX data (Fig.6). However, the diffraction peak correlates to iron oxide which indicates not formed crystal phase of iron oxide with big enough size outside of the porous structure.
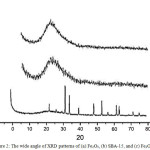 |
Figure 2: The wide angle of XRD patterns of (a) Fe2O3, (b) SBA-15, and (c) Fe2O3/SBA-15 Click here to View figure |
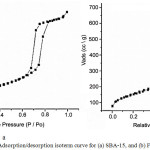 |
Figure 3: Adsorption/desorption isoterm curve for (a) SBA-15, and (b) Fe2O3/SBA-15 Click here to View figure |
The curve of adsorption-desorption isotherm of mesoporous silica SBA-15 and iron modified on mesoporous silica SBA-15 showed by Fig.3. This isotherm generated by pore type of mesoporous silica SBA-15 as same well as IUPAC definition of type IV with H1 as hysteresis loop. The sharp inflection was shifted from P/Po 0.60 to 0.80 indicated the mesopore character range. Pore parameters for SBA-15 dan Fe2O3/SBA-15 are shown in Table 1.
Tabel 1: Pore parameters for SBA-15 dan Fe2O3/SBA-15
| Sample | SBET (m2g-1) | SBJH (m2g-1) |
VBJH (cc g-1) |
DBJH (nm) |
| SBA-15 | 556 | 515 | 1.02 | 8.68 |
| Fe2O3/SBA-15 | 470 | 401 | 0.87 | 6.50 |
The BET surface area of SBA-15 and Fe2O3/SBA-15 decreased from 470 to 556 m2g-1. It also happens in the pore diameter and pore volume. Porosity parameter of asynthesized material such as specific surface area, pore size and volume was decerase due to the blocking pore of molecular sieve SBA-15 by Fe2O3 particles during preparation process. The other reason came to the wet impregnation process that can be a stimulant for the blocking pore. These results indicate that the iron oxide was completely doping onto nanomaterial mesoporous silica SBA-15.
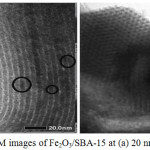 |
Figure 4: TEM images of Fe2O3/SBA-15 at (a) 20 nm and (b) 50 nm Click here to View figure |
Fig 4 show TEM image of Fe2O3/SBA-15 at different magnification. The general TEM image at perpendicular way describe the regular honeycomb framework as mesoporous silica structure. The iron particle which is labelled as black circle in TEM image (Fig 4.a) show that iron has been successfully distributed on the surface of SBA-15. The other information from iron black circle section is the uniform size of disperse iron at range 0.5-1 nm without change the main structure of SBA-15. This result supported by FTIR, XRD, and BET. TEM and XRD data also show that the modification of SBA-15 with iron maintained the uniformity of molecular sieve mesoporous silica SBA-15 that desribed with the hexagonally structure of SBA-15 after all synthesis step.
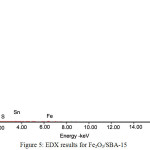 |
Figure 5: EDX results for Fe2O3/SBA-15 Click here to View figure |
Fig 5 show the result of elemental analysis of Fe2O3/SBA-15 preformed by EDX. The elemental identification by EDX explore that of SBA-15 mesoporous silica containing iron particle. Table 2 show that the iron content on Fe2O3/SBA-15 adsorbent is 2,98%.The silica content record up to 50% which indicated that mesoporous silica SBA-15 act as good supporting material to produce Fe2O3/SBA-15. The other fact appear from the S and Sn element that predict as the impurity of precursor of iron. The iron doped within the mesoporous channels of SBA-15 indicates successful impregnation process according to the procedure. The results of EDX characterization is confirmed data from FTIR and XRD.
Table 2: The element content of Fe2O3/SBA-15 by EDX
|
Element |
Weight (%) |
Mass (%) |
|
O |
32.72 |
47.54 |
|
Si |
60.72 |
50.26 |
|
S |
00.49 |
00.35 |
|
Sn |
03.10 |
00.61 |
|
Fe |
02.98 |
01.24 |
Fig 6 show the Adsorption performance of Fe2O3/SBA-15 when adsorbed Nickel ion by conducted in isothermal condition at the room constant temperature. The time influence during nikel ion adsorption on Fe2O3/SBA-15 to adsorption capacityof sample are shown in Fig. 6. The adsorption of Ni (II) affected by the time contacts where the longer contact adsorbents will give the capacity of adsorption increases. The results indicate the optimum adsorption at the time of 240 minutes. Contacting between Fe2O3/SBA-15 as adsorbents and Ni (II) as adsorbate has reached equilibrium and evidenced by the adsorption capacity of samples tend to be constant after 240 minutes.This is due to the adsorption time of 240 minutes, Fe2O3/SBA-15 still active and not saturated yet by Ni (II) solutions.
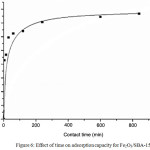 |
Figure 6: Effect of time on adsorption capacity for Fe2O3/SBA-15 Click here to View figure |
In this research, the equation of pseudo-first-order by Lagergren (2) and the pseudo-second-order by Ho and McKay (3) use as model of adsorption kinetic of the Fe2O3/SBA-15. The equation of both model desribed by follow section.
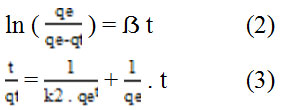
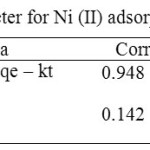 |
Table 3: Kinetics parameter for Ni (II) adsorption onto Fe2O3/SBA-15 Click here to View table |
Where qe (mg/g) is the amount of Ni (II) adsorben upon reaching equilibrum, qt (mg/g) is the amount of Ni (II) adsorben at various times t (min), ẞ (min-1) and k2 (g(mg/min)-1) are the rate constants of the the pseudo-first-order and the pseudo-second-order adsorption kinetic. In Table 3. It shows that the adsorption curve of Ni (II) on Fe2O3/SBA-15 suitable and follows the adsorption kinetics of the Lagergren Model because it has a higher linearity level than the Ho and McKay Models.
Conclusion
The mesoporous silica of SBA-15 has been successfully modified with iron as an adsorbent for adsorption Ni (II) in wastewater. Mesoporous Fe2O3/SBA-15 has a regular amorphous structure obtained under test conditions with XRD instrument. Results show Fe2O3/SBA-15 keep the uniformity of hexagonal molecular sieve of SBA-15 structure after iron doping process. Decreasing pore surface area, pore diameter, and pore volume Fe2O3/SBA-15 compare with SBA-15 indicate that iron particles cover the the mesoporous channels of SBA-15. It also reinforced by EDX result that Fe content in Fe2O3/SBA-15 adsorbents is 2.98 wt%. The functional groups associated with the adsorbent show the Si-OH peak at the wavenumber,1635.71 cm-1 and the Si-O-Si group at the wavenumber, 1084.04 cm-1. The optimum capacity of Ni (II) adsorption reached 240 minutes at an initial Ni (II) concentration of 500 mg L-1. For suggestion, material mesoporous Fe2O3/SBA-15 could be develope as magnetic adsorbent for the future research.
Acknowledgement
Authors thanks the Sebelas Maret University for the funding support by Fundamental Research Grant PNBP Program 2017 (Contract Number 623/UN27.21/PP/2017)
References
- Dhokpande, S. R., Kaware, J. P., and Kulkarni, S. J. International Journal of Science, Engineering and Technology Research, 2013, 2,12, 2278–7798.
- Varma, S., Sarode, D., Wakale, S., Bhanvase, B. A, and Deosarkar, M. P. (2013). International Journal of Chemical and Physical Sciences, 2013, 2,132–139.
- Senthil Kumar, P., Ramakrishnan, K., and Gayathri, R. Journal of Engineering Science and Technology, 2010, 5(2), 234–245.
- Kundra R., Sachdeva R, A. S., and M., P.. J. Curr. Chem. Pharm. Sc, 2012, 2(1), 1–11.
- Turtureanu, A., Georgescu, C., and Oprean, L.. Journal of Applied Sciences and Environmental Management, 2008,10 (3), 159–162. https://doi.org/10.4314/jasem.v10i3.17339.
CrossRef - Amuda, O., Amoo, I., Ipinmoroti, K., and Ajayi. Journal of Applied Sciences and Environmental Management, 2006, 10 (3), 159–162. https://doi.org/10.4314/jasem.v10i3.17339.
CrossRef - Orbély, G. B., and Agy, E. N. Journal of Applied Sciences and Environmental Management, 2008, 10 (3), 159–162. https://doi.org/10.4314/jasem.v10i3.17339.
CrossRef - Srivastava, N. K., and Majumder, C. B. (). Journal of Hazardous Materials, 2008, 151(1), 1–8. https://doi.org/10.1016/j.jhazmat.2007.09.101.
CrossRef - Krishna, R. H., & Swamy, A., Journal of Applied Sciences and Environmental Management, 2011. 1-13.
- Varma, S., Sarode, D., Wakale, S., Bhanvase, B. and Deosarkar, M. P . International Journal of Chemical and Physical Sciences, 2(Special Issue), 2013, 132–139.
- Hoffmann, P. Tomorrow’s Energi: Hydrogen, Fuel Cells, and the Prospects for a Cleaner Planet. 2001,Cambridge: MIT Press.
- Zhao, D., Huo, Q., Feng, J., Chmelka, B. F., and Stucky, G. D. (). J. Am. Chem. Soc., 1998, 120(24), 6024–6036. https://doi.org/Doi 10.1021/Ja974025i.
CrossRef - Taguchi, A. and Schuth F., Microporous Mesoporous Mater., 2004, 77, 1-45.
CrossRef - Zhao, D., Wan, Y., dan Zhou, W., Ordered Mesopororous Material. 2013, Germany:Wiley.VchVerlag GmbH and Co. KgaA.
- Zhao, J., Gao, F., Fu, W., Jin, W., Yang, P., and Zhao, D., Chemical Communications, 2002, 7, 752-753.
CrossRef - Zhang, Y., Liu, R., Hu, Y., and Li, G. Chemical Communications, 2009. 81(3), 967–976. https://doi.org/10.1021/ac8018262.
CrossRef - Deng, Y., Yang, W., Wang, C., and Fu, S., Advanced Materials, 2003, 15(20), 1729–1732. https://doi.org/10.1002/adma.200305459.
CrossRef - Huang, H., Ji, Y., Qiao, Z., Zhao, C., He, J., and Zhang, H., Journal of Automated Methods & Management in Chemistry, 2010,323-339. https://doi.org/10.1155/2010/323509
CrossRef - Vijayaraghavan, K., Jegan, J.R., and Velan, M., Journal of Biotechnology, 2004, 7(1), 61-71.
- Viart, N., & Rehspringer, J.L. Journal of Biotechnology, 2009, 12(1), 1-11.
- Amama, P.B., Lim, S., Ciuparu, D., Yang, Y., Pfefferle, L., and Haller, G.L., Journal Physics Chemistry. 2005, 109(7), 2645-2656.
CrossRef - Antonio, T.J.A., Jacome, C.M.A., Cerros, O.S.L., Palacios, M.E., Parra, S.R., Chavez, A.C., Navarete, J., &Salinas, L.E., Applied Catalysis B: Environmental. 2010, 100, 47-54.
CrossRef - Sing, K.S.W., Everett, D.H., Haul, R.A.W., . Pure Applied Chemistry. 1985, 57(4), 603-619.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









