Synthesis and Cytotoxic Activities of Hexyl Esters Derivatives of Gallic Acid Against MCF-7 Cell line
Rafika Indah Paramita1, Ade Arsianti1 and Maksum Radji2
1Department of Medical Chemistry and Drug Development Research Cluster, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia.
2Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia.
Corresponding Author E-mail: arsi_ade2002@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340132
Gallic acid is a compound that found in many plants, fruits, and foods where the anti-cancer activity is the best activity. However, gallic acid has a problem on the high polarity and low bioavailability. So, it takes molecular modifications in order to increase its lipophilicity, which is expected to increase bioavailability and cytotoxic activity of gallic acid. Hexyl esters derivatives of gallic acid were synthesized and characterized by spectrometer 1H-NMR, 13C-NMR, mass spectrometry and infrared spectrophotometer (FTIR). All compounds were then evaluated for cytotoxic activity on MCF-7 cell line using MTT method. Compound cis-2’-hexenyl-3,4,5-trimethoxygallate (19) had the lowest IC50 value compared with gallic acid and other derivatives hexyl esters. IC50 value of cis-2’-hexenyl-3,4,5-trimethoxygallate (19) is 14.48 µg/ml. Compound (19) also has approached with IC50 values of gossypol as a positive control. Compound (19) is a potential compound to inhibit growth of MCF-7 cell line.
KEYWORDS:Gallic Acid; MCF-7; cytotoxicity Assay; Synthesis of Hexyl Esters Gallate Derivative
Download this article as:| Copy the following to cite this article: Paramita R. I, Arsianti A, Radji M. Synthesis and Cytotoxic Activities of Hexyl Esters Derivatives of Gallic Acid Against MCF-7 Cell line. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Paramita R. I, Arsianti A, Radji M. Synthesis and Cytotoxic Activities of Hexyl Esters Derivatives of Gallic Acid Against MCF-7 Cell line. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=43131 |
Introduction
Breast cancer is the second most common cancer in the world and the most frequent cancer among women with an estimated 1.67 million new cancer cases diagnosed in 2012 (25% of all cancers). Breast cancer ranks as the fifth cause of death from cancer overall.1 A lot of research to synthesis new potential anticancer drugs. Although many potential anticancer drugs have been made but the medical need is still largely unmet due to many factors among which the lack of selectivity of conventional drugs leading to toxicity, the metastatic spreading, and multi-drug resistance; MDR. Therefore, the search for novel and selective anticancer agents is highly required due to currently available anticancer drugs problems.2,3
In recent decades, the development of novel and advanced cancer therapies common use natural plants, fruits, or foods as a valuable resource. Gallic acid (3,4,5-trihydroxyl-benzoic acid) is compound which is widely distributed in various plants, fruits, and foods. Gallic acid was demonstrated to have various biological activities including antibacterial, antiviral, and anti-inflammatory, in which the antitumor activity is most striking.3 Growth of human breast cancer cell, MCF-7, is significantly reduced by treatment with gallic acid. Gallic acid causes apoptosis induction and cells treated with gallic acid showed significantly downregulated of Bcl-xL protein and upregulation of Bak and Bad proteins.4
In previous study, we were done with in silico study about hexyl esters derivatives of gallic acid. Hexyl esters of trimethoxygallic acid showed more interaction of hydrophobic amino acid than gallic acid.5 This leads to increased bond stability with the receptor. Compound cis-2’-hexenyl-3,4,5-trimethoxygallate serve as potential inhibitor of anti-apoptotic Bcl-xL protein because had the lowest ΔG dan Ki. There is also pi-pi interaction with TYR105 which adds stability to protein-inhibitor interactions and protein complexes.6
To prove the docking result, we had synthesized the hexyl esters derivative of gallic acid (Figure 1) and further testing the cytotoxic activity against MCF-7 cell line. The in vitro result then compared to in silico docking result to get a potent anticancer drug for breast cancer.
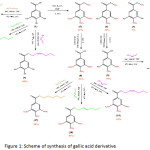 |
Figure 1: Scheme of synthesis of gallic acid derivative Click here to View figure |
Experimental
Synthesis Reaction
Chemicals, reagents and solvents were purchased from Merck and Sigma-Aldrich. 1H-NMR (proton nuclear magnetic resonance) was measured using JEOL JNM-ECP (500 Hz) in Acetone-d6 as solvent. 13C-NMR (carbon nuclear magnetic resonance) was measured using JEOL JNM-ECP (125 Hz) in Acetone-d6 as solvent. The chemical shifts were reported in ppm and coupling constants were measured in Hertz (Hz). Reactions were monitored with thin layer chromatography using aluminum sheets silica gel 60 F254 2×5 cm (Merck). Ultra-violet light (254 nm) and iodine vapour were used to visualized the chromatograms. Mass spectra (MS) were recorded on Shimadzu GCMS QP-5000, IR spectrum were recorded using IR (JASCO FT/IR-420 spectrophotometer.
Methylation Reaction
A solution of gallic acid (17.65 mmol), and K2CO3 (52,83 mmol) in DMF (25 mL) were added Methyl iodide (52.83mmol) and stirred at room temperature for 48h. The reaction mixture was then added 75 ml ethyl acetate and then washed with 3×25 ml saturated NaHCO3 and 2×25 ml saturated NaCl, sequently. The organic phase was then added Mg2SO4 anhydrate to take out the water residue. The filtrate was then drying to obtain the crude product. The product was purified using chromatography colomn with chloroform as mobile phase and give 3 products : 4-monomethoxymethyl gallate, 4,5-dimethoxymethyl gallate, 3,4,5-trimethoxymethyl gallate.
Hidrolysis Reaction
A solution of each methylation product and LiOH monohydrate 0.5 M in mixture THF:Methanol (3:1) were stirred at room temperature for 24h. The reaction mixture was then acidified using KHSO4 1M until pH=3, then added 15 mL of aquadest, and extracted with 3×15 ml ethyl acetate. The organic phase was washed with saturated NaCl and then added Mg2SO4 anhydrate to take out the water residue. The filtrate was then drying to obtain the product and give 3 products: 4-monomethoxygallic acid, 4,5-dimethoxygallic acid, 3,4,5-trimethoxygallic acid, respectively.
Esterification Reaction
A solution of gallic acid or 3,4,5-trimethoxygallic acid (1 mmol), alcohols (2 mmol) and 1.5 mmol Diisopropyl carbadiimide (DIC) as catalisator in Tetrahydofurane (10 mL) were stirred in 00C for 30 minutes. Then added solution of 0,1 mmol N,N-Dimethylaminopiridine (DMAP) in 1 ml THF. This mixture was stirred at room temperature for 24h. The reaction mixture was then added aquadest and extracted with chloroform. The organic phase was then added Mg2SO4 anhydrate to take out the water residue. The filtrate was then drying to obtain the crude product. The product was purified using chromatography colomn with appropriate mobile phase.
Cytotoxicity Effects
The inhibitory effect of synthesized compounds against colorectal adenocarcinoma cell line, HT-29, was determined using MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. First, cells were seeded in 96-well plates at 5000 cells/well and allowed to attach in 24 h. Media was renewed and the cells were added with several concentrations of the extract (3.125–100 ppm) incubated for an additional 24 h. After 24 h, 20 µM of MTT solution (0.5 mg/ml MTT solution in media) was added to each well and incubated for 4 h at 370C. The supernatant was aspirated and the MTT-formazan crystals formed by metabolically viable cells were dissolved in 100 µl of dimethyl sulphoxide (DMSO). Finally, the absorbance was monitored by a microplate reader at a wavelength of 570 nm. The percentage of viable cells was plotted versus the concentration of the test compound. The concentration by which to mediate 50% cytotoxicity (IC50) was determined by linear regression analysis.
Result and Discussion
Synthesis of the desired compounds
The compounds were synthesized as per figure 1 and the structure was elucidated by spectroscopic data, i.e IR, 1H-NMR, 13C-NMR and LCMS are presented as follows:
Hexylgallate (2)
IR (KBr): v cm-1, 3326.61 (OH), 2927.41 (Al. C–H), 1685.48 (C=O), 1535.06 (Ar C=C), 1222.65 (C–O); 1H NMR (Acetone-d6) d: 7.11 (s, 2H, Ar-CH), 4.19 (t, 2H, OCH2CH2), 1.74 – 1.66 (m, 2H, CH2CH2CH2), 1.47 – 1.39 (m, 2H, CH2CH2CH2), 1.36 – 1.30 (m, 4H, CH2CH2CH2), 0.89 (t, 3H, CH2CH3). 13C NMR (Acetone-d6) d: 166.72, 146.02 (2C), 138.48, 122.03 (2C), 109.64, 64.91, 42.04, 32.18, 26.41, 23.59, 14.24. LCMS m/z: [M+]254.1154 (C13H18O5).
trans-2’-hexenylgallate (3)
IR (KBr): v cm-1, 3272.61 (OH), 2927.41 (Al. C–H), 1685.48 (C=O), 1535.06 (Ar C=C), 1226.5 (C–O); 1H NMR (Acetone-d6) d: 7.08 (s, 2H, Ar-CH), 5.84 – 5.76 (m, 1H, CH2CH=CH2), 5.67 – 5.59 (m, 1H, CH=CHCH2), 4.61 (dd, 2H, OCH2CH2), 1.41 – 1.31 (q, 2H, CH2CH2CH2), 1.10 – 1.02 (s, 2H, CH2CH2CH3) 0.86 (t, 3H, CH2CH3). 13C NMR (Acetone-d6) d: 166.50, 146.08 (2C), 136.03, 131.22, 125.56 (2C), 109.66 (2C), 65.46, 42.18, 23.53, 13.82. LCMS m/z: [M+]252.0998 (C13H16O5).
cis-2’-hexenylgallate (4)
IR (KBr): v cm-1, 3326.61 (OH), 2927.41 (Al. C–H), 1685.48 (C=O), 1535.06 (Ar C=C), 1222.65 (C–O); 1H NMR (Acetone-d6) d: 7.01 (s, 2H, Ar-CH), 5.65 – 5.54 (m, 2H, CH2CH=CH), 4.72 (d, 2H, OCH2CH2), 2.14 – 2.06 (q, 2H, CH2CH2CH2), 1.42 – 1.32 (m, 2H, CH2CH2CH3) 0.92 (t, 3H, CH2CH3). 13C NMR (Acetone-d6) d: 166.52, 157.91, 146.77 (2C), 135.11, 125.14 (2C), 109.59 (2C), 60.37, 23.13, 21.43, 13.85. LCMS m/z: [M+]252.0997 (C13H16O5).
cis-2’-hexenyl-3,4-dimethoxygallate (16)
IR (KBr): v cm-1, 3403.74 (OH), 2956.34 (Al. C–H), 1683.35 (C=O), 1587.13 (Ar C=C), 1324.86 (C–O); 1H NMR (Acetone-d6) d: 7.20 (s, 1H, Ar-CH), 7.16 (s, 1H, Ar-CH), 5.73 – 5.64 (m, 2H, CH2CH=CH), 4.83 (d, 2H, OCH2CH2), 3.89 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 2.20 – 2.15 (q, 2H, CH2CH2CH2), 1.47 – 1.39 (s, 2H, CH2CH2CH3) 0.93 (t, 3H, CH2CH3). 13C NMR (Acetone-d6) d: 166.10, 163.15, 153.75, 135.56, 132.24, 126.31, 124.68, 105.49, 60.96, 60.59, 58.21, 29.65, 23.06, 13.72. LCMS m/z: [M+]280.1311 (C15H20O5).
hexyl-3,4,5-trimethoxygallate (17)
IR (KBr): v cm-1, 2935.13 (Al. C–H), 1714.41 (C=O), 1589.06 (Ar C=C), 1224.58 (C–O); 1H NMR (Acetone-d6) d: 7.30 (s, 2H, Ar-CH), 4.29 (t, 2H, OCH2CH2), 3.88 (s, 6H, OCH3), 3.79 (s, 3H, OCH3), 1.79 – 1.72 (m, 2H, CH2CH2CH2), 1.49 – 1.42 (m, 2H, CH2CH2CH2), 1.38 – 1.33 (m, 4H, CH2CH2CH2), 0.90 (t, 3H, CH2CH3). 13C NMR (Acetone-d6) d: 166.69, 154.45 (2C), 143.63, 126.73, 107.86 (2C), 65.91 60.94, 56.79 (2C), 32.49, 26.71, 23.52 (2), 14.57. LCMS m/z: [M+]296.1624 (C16H24O5).
trans-2’-hexenyl-3,4,5-trimethoxygallate (18)
IR (KBr): v cm-1, 2958.27 (Al. C–H), 1714.41 (C=O), 1589.06 (Ar C=C), 1222.65 (C–O); 1H NMR (Acetone-d6) d: 7.24 (s, 2H, Ar-CH), 5.87 – 5.77 (m, 1H, CH2CH=CH), 5.70 – 5.58 (m, 1H, CH=CHCH2), 4.69 (dd, 2H, OCH2CH2), 3.82 (s, 6H, OCH3), 3.74 (s, 3H, OCH3), 2.04 – 2.02 (q, 2H, CH2CH2CH2), 1.42 – 1.32 (m, 2H, CH2CH2CH3) 0.85 (t, 3H, CH2CH3). 13C NMR (Acetone-d6) d: 166.47, 154.46 (2C), 143.69, 136.91, 126.59, 125.65, 107.91 (2C), 64.45, 60.94, 56.81 (2C), 35.27, 23.09, 14.17. LCMS m/z: [M+]294.1467 (C16H22O5).
cis-2’-hexenyl-3,4,5-trimethoxygallate (19)
IR (KBr): v cm-1, 2958.27 (Al. C–H), 1714.41 (C=O), 1589.06 (Ar C=C), 1220.72 (C–O); 1H NMR (Acetone-d6) d: 7.29 (s, 2H, Ar-CH), 5.73 – 5.61 (m, 2H, CH2CH=CH), 4.85 (d, 2H, OCH2CH2), 3.86 (s, 6H, OCH3), 3.78 (s, 3H, OCH3), 2.20 – 2.15 (q, 2H, CH2CH2CH2), 1.47 – 1.38 (m, 2H, CH2CH2CH3), 0.92 (t, 3H, CH2CH3). 13C NMR (Acetone-d6) d: 166.24, 154.14 (2C), 143.38, 135.77, 126.25, 124.85, 107.60 (2C), 61.29, 60.64, 56.49 (2C), 29.84, 23.24, 13.92. LCMS m/z: [M+]294.1465 (C16H22O5).
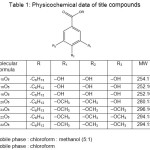 |
Table 1: Physicochemical data of title compounds Click here to View table |
After completion of the synthesis, cytotoxic activity of gallic acid, target derivative 2, 3, 4, 16, 17, 18, and 19 were determined MTT cell proliferation assay against MCF-7 cell line, cytotoxic activity represented by IC50. The smaller IC50 value, the higher cytotoxic activity. From the MTT assay result, compound 19 showed the best cytotoxic activity than other compound, and the IC50 value of compound 19 similar to IC50 value of Gossypol as positive control. This result was agreevwith in silico study in previous research by Paramita, 2017. Cytotoxicity assay of gallic acid derivative are summarized in table 2 as follow.
Table 2: Cytotoxic activity of hexyl esters derivatives of gallic acid
| Compound | IC50 (µg/ml) |
| Gallic acid | 32.25 ± 0.014 |
| 2 | 24.38 ± 0.014 |
| 3 | 21.28 ± 0.030 |
| 4 | 20.80 ± 0.087 |
| 16 | 18.93 ± 0.062 |
| 17 | 16.54 ± 0.012 |
| 18 | 14.93 ± 0.000 |
| 19 | 14.48 ± 0.087 |
| Gossypol | 10.90 ± 0.000 |
As shown in table 2, seven derived compounds had greater inhibitory activity against MCF-7 cell line than gallic acid. Gallic acid has an IC50 value of 32.25 μg / ml. The results are similar to IC50 of gallic acid in previous studies, i.e. 36 μg / ml.7 Increased lipophilicity of the gallic acid derivative (alkyl ester derivative) will increase the cytotoxic activity. In the previous study, it was shown that alkyl esters of gallic acid had better cytotoxic effects than gallic acid. This is due to an increase in lipophilicity that contributes in increasing affinity for cell membrane and permeability.8 IC50 values of hexylgallate 2, trans-2′-hexenylgallate 3, and cis-2′-hexenylgallate 4 are 24.38 μg / ml, 21.28 μg / ml, 20.80 μg / ml, respectively, where the value is lower than the gallic acid.
In the double-bonded hexenyl ester compound, both the cis 4 and trans 3 positions exhibit lower IC50 than the hexyl ester compound without the double bond 2. This suggests that the presence of double bonds may increase the cytotoxic activity of the compound. In addition, double bond positions with cis configurations in compounds 4 and 19 exhibit slightly better activity than trans configurations in compounds 3 and 18. In ester compounds without methoxy groups, ie hexylgallate 2, trans-2′-hexenylgallate 3, and cis-2′-hexenylgallate 4, showed less cytotoxic activity than those with methoxy groups. In compounds with the same alkyl ester, cis-2′-hexenyl ester, in compounds 2, 16, 19, with differences in the number of methoxy groups, also shows increased cytotoxic activity as the number of methoxy groups increases. The bonding sites of the Bcl-xL protein are mostly composed of non-polar amino acids.9 Thus the methoxy group has a more stable bond on the protein binding site of Bcl-xL compared with the hydroxy group.
From the MTT test results, it is known that the cis-2′-hexenyl-3,4,5-trimethoxygallate 19 compound exhibited the best activity with the lowest IC50 value compared to the other gallic acid derivative compounds and the results obtained supported the previous results of in silico study by Paramita et al, 2017. Compound 19 also had an IC50 value close to the IC50 value of gossypol as a positive control. Compound 19 is a potential compound in inhibiting growth of MCF-7 cell line because has the best in vitro activity between synthesized gallic acid derivatives.
Conclusion
We described synthesis of hexyl esters derivatives of gallic acid for in vivo cytotoxic activities against MCF-7 cell line. Among all the compounds synthesized, compounds 19 exhibited most potent cytotoxic activity and had an IC50 value close to the IC50 value of gossypol. Based on the findings of these in vitro results, further studies need to be carried out to investigate in vivo assays and toxicological studies.
Acknowledgements
This article does not contain any studies with human and animal subjects performed by any of the authors. We thank to Faculty of Medicine, Universitas Indonesia for the Cluster Research Grant 2017.
References
- Ferlay, J.; Soerjomataram, Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M. Intl. J. of Cancer. 2015,136 (5).
- Shukla, S.; Srivastava, R.S.; Shrivastava, S. K.; Sodhi, A.; Kumar, P. Asian Pac. J. Trop. Biomed. 2012, 5, 1040–46.
CrossRef - Jaikumar, B. & Jasmine, R. Int. J. PharmTech Res. 2016, 9, 333–365.
- Wang, K.; Zhu, X.; Zhang, K.; Zhu, L.; Zhou, F. J. Biochem. Mol. Tox. 2014, 28(9), 387–93.
CrossRef - Paramita, R.I.; Arsianti, A.; Radji, M. Intl. J. ChemTech Res. 2017, 1, 385.
- Shipra, G.; Gauri, M.; Chandra, P.M.; Kishore, S.P. Prot. & Pept. Let.. 2012, 19,1302–17.
CrossRef - Mukherjee, P.; Desai, P.; Zhou, Y. D.; Avery, M. J. Chem. Inform. Modeling. 2010, 50(5), 906–923.
CrossRef - Tor, Y. S., et al. PLoS ONE. 2015, 10(6), 1–25.
CrossRef - Locatelli, C.; Filippin-Monteiro, F. B.; Creczynski-Pasa, T. B. Euro. J. Med. Chem. 2013, 60, 233–239.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









