Synthesis and Characterization of some new β-Lactam Derivatives from Azo Sulphadiazine and its Biological Evaluation as Anticancer
Radhiyah A. Khdur1 and Ezzat H. Zimam2
and Ezzat H. Zimam2
1Department of Chemistry, Faculty of Education for Girls, the University of Kufa / Iraq.
2Department of Chemistry, Faculty of Science, the University of Kufa / Iraq.
Corresponding Author E-mail: radhiyah.aldujaili@uokufa.edu.iq
DOI : http://dx.doi.org/10.13005/ojc/340140
In this work new Azo compound was synthesized through a diazo-coupling reaction (S1) (4-amino-N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl)benzene sulfonamide). The diazotization process of 2-aminopyrimidin with Sulphadiazine as coupling component was described, then schiff bases [B1-B5] prepared from condensation of the free amino group in the azo compound with many aromatic aldehydes by using glacial Acetic acid as solvent. cyclization of Shiff bases with Chloro acetyl chloride in the presence of tri ethyl amine give the corresponding β-lactam derivatives [L1-L5]. All these compounds identified by( FT-IR,1H-NMR, 13C-NMR) and follow reaction by (TLC) and Measurement melting points of some them.
KEYWORDS:2-Amino Pyrimidine; Sulphadiazine; Schiff Base; Β-Lactam
Download this article as:| Copy the following to cite this article: Khdur R. A, Zimam E. H. Synthesis and Characterization of some new β-Lactam Derivatives from Azo Sulphadiazine and its Biological Evaluation as Anticancer. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Khdur R. A, Zimam E. H. Synthesis and Characterization of some new β-Lactam Derivatives from Azo Sulphadiazine and its Biological Evaluation as Anticancer. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41794 |
Introduction
Sulfadiazine is a sulfonamide antibiotic and it is recognized as one of” the World Health Organization’s List of Essential Medicines”, itremoves bacteria that cause infections by stopping the production of folic acid into the bacterial cell, and is Usually used to” treat urinary tract infections” (UTIs) and burns [1,2]. Schiff bases are Compounds containing an azo methine group (-CH=N)which formed by the condensation of an active carbonyl compound with primary amine ,this reaction is recognized to be reversible and the removal of water to drive the reaction to completion is often fundamental to gain a good yield [3,4,5]. Schiff bases are the important compounds due to their wide range of industrial application and biological activities. they have been reported to posses the pharmacological activities such as antibacterial, anti-malarial, anti-fungal, antioxidant , anti-cancer, anti-tubercular, anti-inflammatory, insecticidal and anticonvulsant . also used as versatile component with organo-metallic reagents in nucleophilic addition and in cyclic addition reactions [6.7,8]. β-lactam (2-Azetidinone) are four-membered cyclic amide that which derived from 3-amino-propanoic acid [9]. It is named so because the (N) atom is connected to the β-carbon atom close to the carbonyl [10]. The β-lactam ring is portion of the core structure of several antibiotic families, the major ones being the cephalosporins, penicillins, carbapenems, and monobactams, which are also called (β-lactam antibiotics). Almost all of these antibiotics work by inhibition bacterial cell wall biosynthesis [11]. The first synthetic β-lactam ring was prepared by Hermann Staudinger in (1907) by reaction of the Schiff base ofbenzaldehyde and aniline with diphenylketene in a [2+2] cycloaddition [12,13]. A new research has suggested that β-lactams can subject ring-opening polymerization to form the amide bonds, to become “nylon-3 polymers”. The basis of these polymers are similar to peptides, which offer them biofunctionality. nylon-3 polymers can either mimic host defense peptides or act as signals to stimulate 3T3 stem cell function [14].
This research involve synthesis of some new β-lactam derivatives mixed with azo compound and study biological activity of the prepared compounds.
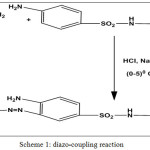 |
Scheme 1: diazo-coupling reaction Click here to View scheme |
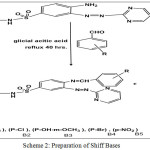 |
Scheme 2: Preparation of Shiff Bases Click here to View scheme |
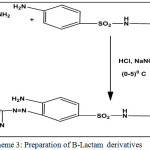 |
Scheme 3: preparation of B-Lactam derivatives Click here to View scheme |
Experimental
All materials were of highest purity and supplied by Merck and Fluka- company. Measurements melting points were recorded by using electro thermal 9300 ,” melting point engineering LTD , U.K”. Thin layer chromatography (T.L.C)was performed on silica gel for (T.L.C) and spots were visualized by Iodine vapors.” FT-IR” spectra ,Fourier transform infrared shimadzu (8400), H1-NMR & C13-NMR-spectra in (ppm) unit were operating in DMSO –d6 as solvent using (Bruker- Ultra Shield 300 MHz Switzerland)- (Iran).
Synthesis of 4-Amino-N-(Pyrimidine-2-yl)-3-( Pyrimidine-2-yldiazenyl)benzenesulfonamide(Azo)(S1 )[19]
2- amino Pyrimidine (0.01mole) (0.95gm) was dissolved in (4ml) of concentrated hydrochloric acid and (15 ml) of distilled water The mixture was cooled at (0-50C) in ice-water bath ,Then a solution of sodium nitrite (0.01 mole) (0.69 gm ) was dissolved in (10 ml) of distilled water then it will be cooled at (0-50C). This solution was added a drop wise to the mixture with stirring at the same temperature. The diazonum salt solution was added portion wise to solution of (0.01 mol, 2.5 gm) sulfadiazine in distilled water with sodium hydroxide (5 gm) dissolved in (100ml) distilled water. The basicity was nutralise by adding drops of (HCl ) until the PH become (7) and temperature was maintained at (0-5°C).The mixture was stirred for 30 mint and was left over night. The product was precipitated and filtered, washed well with distilled water and re-crystallized from absolute ethanol ,yield (yellow)( 89.5%) m.p . (248-249°C).
I.R spectra, (NH2 ) str. (3427-3358)cm-1, Azo (1440-1492) cm-1, (C=C) str.Aromatic (1585) cm-1, (N-H) str. Sulfone (3257)cm-1, (C=N) str. Pyrimidine(1666-1635) cm-1, (C-H) str. Aromatic (3039) cm-1, SO2 (1325) cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz), (NH2) (6.015) (Ar-H) (6.559-7.033), (HC=N) pyrimidine (7.612-8.498), (N-H) Sulfone (11.294), DMSO(2.5).
C13-NMR-spectrum, (δ ppm), (DMSO-d6,MHz), 110.56, 112.59 , 115.99, 125.33, 130.28, 153. 50, 157.69, 158.47, 158. 71.
General procedure for synthesis Schiff bases [B1-B5][20]
Azo Compound (S1) (0.01 mol) was dissolved in hot glacial acetic acid about (50 ml) then added to (0.01 mol) of different aromatic aldehydes were dissolved in (5 ml) of glacial acetic acid. The reaction mixture was refluxed at (100°C) with stirring for (40) hours. The progress of the reaction was followed by TLC .After the completion the mixture was poured onto ice crushed. The yielded solid was filtered off and wash with (2%)Sodium bicarbonate solution and distilled water then re-crystallized from abs. Ethanol.
4-(dimethylamino)benzylideneamino)-N-(pyrimidine-2-yl)-3-(pyrimidine-yldiazenyl)benzenesulfonamide[B1]:
(3.56 gm) of Azo comp, (S1) with (1.49gm) from (p- N,N-dimethyl amino benzaldehyde), the color of yield (red), (72%),m.p.(150-152°C), T.L.C. (MET:TOL) (6:4) (Rf, 0.92 ).
I.R spectra ( C=N ) str. Imine (w 1640) cm-1, (S .C=N ) Pyrimidine (1678)cm-1, (C=C) str.Aromatic (1533-1585) cm-1, (N-H) str. Sulfone (3311)cm-1, (C=N) str. Pyrimidine(w. 1630) cm-1, AZO(1440-1494) cm-1, Aliphatic C-H(2931) cm-1, (C-H) str. Pyrimidine ring(3184) cm-1,, (C-H) str. Aromatic(3037-3111) cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz) (N-CH3)2 (3,058), (H-C=N)Imine(8.518) (Ar-H) (6.55-7.979), (HC=N) pyrimidine (8.002-8.49), (N-H) Sulfone ( 11.279), DMSO(2.5).
C13-NMR-spectrum, (δ ppm), (DMSO-d6, MHz), 31.7, 111.55, 112.58, 115.99, 125.32, 127.91, 130.28 , 132.03 , 153.48, 157.69, 158.73.
4-(chlorobenzylideneamino)-N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl) benzenesulfonamide[B2]
(3.56 gm) of Azo comp,(S1) with (1.405gm) from (p-Chloro benzaldehyde), the color of yield (Brown),(60%), m.p. (166-168°C), T.L.C. (MET:TOL) (6:4) (Rf, 0.82).
I.R spectra ( C=N )str.Imine(w. 1640)cm-1, (C=C) str.Aromatic (1541-1585) cm-1, (N-H) str. Sulfone (3311)cm-1, (C=N) str. Pyrimidine(S. 1680) cm-1, Azo(1419-1494) cm-1, (C-H) str. Pyrimidine ring(3186) cm-1, (C-H) str. Aromatic(3037-3111) cm-1, (C-Cl)(615 cm-1).
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz) ,(H-C=N)Imine(8.768) (Ar-H) (7.050-7.945), (HC=N) pyrimidine (8.035-8.536), (N-H) Sulfone ( 11.079), DMSO(2.5).
C13-NMR-spectrum, (δ ppm) ,(DMSO-d6,MHz),116.11 , 118.657 , 129.352, 134.276 , 143.641 , 157.458 , 158.809,169.531.
(4-hydroxy-3-methoxybenzylidineamino)–N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl) benzenesulfonamide [B3]
(3.56 gm) of Azo comp, (S1) with (1. 52gm) from Vaniline (p-hydroxy-m-methoxy benzaldehyde), the color of yield (Orange), (61%),m.p.(145-146°C), T.L.C. (MET:TOL) (3:2) (Rf, 0.87).
I.R spectra (C=N) str. Imine (1639)cm-1, (C=N) Pyrimidine (1674) cm-1, (C=C) str. Aromatic (1577) cm-1, (N-H) str. Sulfone (3377)cm-1, (C=N) str. Pyrimidine (1639) cm-1,AZO(1438-1492) cm-1, , (C-H) str. Pyrimidine ring(3257) cm-1, (C-H) str. Aromatic(3037-3105) cm-1, (OH)(3458 cm-1), Aliphatic C-H(2937) cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz), (H-C=N) Imine (8.66), (O-CH3) (3.919), (Ar-H) (6. 263-7.247), (HC=N) pyrimidine (7.551-8.446), (N-H) Sulfone (11.47), (OH) (9.77), DMSO (2.5).
C13-NMR-spectrum, (δ ppm), (DMSO-d6, MHz), 56.017, 111.047, 112.583, 115.828, 115.992, 116.168, 118.642, 125.248, 126.603, 129.148, 129.339, 130.309, 134.306, 143.622, 148.603, 153.515, 157.486, 157.679, 158.732, 158.810,169.525.
4-(Bromo benzylideneamino)-N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl) benzenesulfonamide[B4]
(3.56 gm) of Azo comp, (S1) with (1. 84gm) from (p-bromo benzaldehyde), the color ofyield (darkgoldenrod), (63%), m.p. (173-175°C), T.L.C. (MET:TOL) (1:4) (Rf, 0.55).
I.R spectra (C=N) str. Imine (w.1645) cm-1, (C=N) str. Pyrimidine (m.1672)cm-1, (C=C) str. Aromatic (1579) cm-1, (N-H) str. Sulfone (3271)cm-1, AZO(1415-1448) cm-1, (C-H) str. Pyrimidine ring(3186) cm-1, (C-H) str. Aromatic(3037-3105) cm-1, (C-Br)798 cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz) ,(H-C=N) Imine (8.840), (Ar-H) (6.889-7.945), (HC=N) pyrimidine (8.053 -8. 715), (N-H) Sulfone ( 11.256), DMSO(2.5).
C13-NMR-spectrum, (δ ppm), (DMSO-d6, MHz), 113.706, 115.511, 118.562, 118.961, 123.694, 125.248, 129.192, 129.880, 130.938, 131.741, 132.469, 143.285, 143.605, 146.942, 158.633, 160.125, 169.435.
4-(Nitro benzylideneamino)-N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl)benzenesulfonamide[B5]
(3.56 gm) of Azo comp, (S1) with (1. 51gm) from (p-Nitro benzaldehyde), the color of yield (orange), (76%), m.p. (202-204°C), T.L.C. (MET:TOL) (2:3) (Rf, 0.64).
I.R spectra ( C=N ) str. Imine interaction with (C=N) Pyrimidine (1680)cm-1, (C=C) str. Aromatic (1585) cm-1, (N-H) str. Sulfone (3311)cm-1, AZO (1406-1440) cm-1, (C-H) str. Pyrimidine ring(3182) cm-1, (C-H) str. Aromatic(3039-3113) cm-1, (NO2 )(1336-1535) cm-1 .
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz), (H-C=N) Imine (S 8.893), (Ar-H) (m 7.068-7.981), (HC=N) pyrimidine (m 8.339-8.643 , 9.233), (N-H) Sulfone (11.363), DMSO(2.5).
C13-NMR-spectrum, (δ ppm) ,(DMSO-d6, MHz), 116.421, 118.348, 122.842, 124.554, 129.476, 131.188, 132.044, 133.756, 143.815,157.939, 159.651, 169.710.
Preparation of β-Lactam derivatives[L1-L5] from Schiff bases[21]
To a mixture of Schiff base (0.01 mol) in dioxane (25 ml)and triethylamine (3.49 ml, 0.025 mol), was added chloro acetyl chloride (1.99 ml, 0.025 mol) drop-wise at( 5-10) °C. The reaction mixture was stirred for (6 hrs) and kept at room temperature for two days then poured into crushed ice. The solvent was evaporated and the yalid recrystallized from ethanol. All the reactions was monitored by (T.L.C).
4-(3-chloro-2-(4-(dimethylamino)phenyl)-4-oxoazitidine-1-yl)-N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl)
benzenesulfonamide[L1]:
Was prepared from shiff base [B1] (4.87 gm, 0.01 mole), Et3 N (3.49 ml,0.025 mole) and ClCH2COCl (1.99 ml, 0.025 mole), the color (Brown), 63%, m.p. (Gummy), T.L.C. (MET:TOL ) (1:4) (Rf, 0.41).
I.R spectra ( C=O )str. (S.1743)cm-1, (C=N) Pyrimidine (1662), (C=C) str. Aromatic (1595) cm-1, (N-H) str. Sulfone (3404)cm-1, AZO (1436-1463) cm-1, (C-H) str. Aliphatic (2854-2929)cm-1, (C-Cl) 572cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz), (N-CH3) (S 2.827), (N-CH) (d.5.440),(CO-CH-Cl) (d.5.85), (Ar-H) (7.516-8.142), (HC=N) pyrimidine (8.607-9.325), (N-H) Sulfone (11.184), DMSO(2.5).
C13-NMR-spectrum, (δ ppm), (DMSO-d6, MHz), 46, 62, 64, 116.64, 118.78, 121.77, 125.84, 127.12, 129.05, 133.33, 135.25, 139.53, 141.67, 157.08, 159.22, 169.71, 172.
4-(3-chloro-2-(4-(chloro phenyl)-4-
oxoazitidine-1-yl)-N-(pyrimidine-2-yl)-3-(pyrimidine-2-yldiazenyl)benzenesulfonamide[L2]:
Was prepared from shiff base [B2] (4.785 gm, 0.01 mole), Et3 N (3.49 ml,0.025 mole) and ClCH2COCl (1.99 ml,0.025 mole), the color (pal yallow), 50%, m.p. (Oily), T.L.C. (MET:TOL) (1:4) (Rf, 0.60).
I.R spectra (C=O) str. (S.1741)cm-1, (C=C) str.Aromatic (1595) cm-1, (N-H) str. Sulfone (3415)cm-1, AZO (1462) cm-1, (C-H) str. Aliphatic (2937-2981) cm-1,(C-Cl) (w.582)cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz) ), (N-CH) (d.3.740), (CO-CH-Cl) (d.4.062), (Ar-H) (7.713-8.607), (HC=N) pyrimidine (8. 786-9.341), (N-H) Sulfone (10.934).
C13-NMR-spectrum, (δ ppm), (DMSO-d6, MHz), 58.211, 68.269, 110.429, 114.281, 117.277, 121.558, 124.982, 129.690, 130.974, 134.826, 139.320, 145.313, 149.379, 151.519, 161.149, 165.002, 172.706, 182.122
4-(3-chloro-2-(4-(hydroxyl-3-methoxy phenyl)-4-oxoazitidine-1-yl) -N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl) benzenesulfonamide[L3]
Was prepared from shiff base [B3] (4.9 gm, 0.01 mole), Et3 N(3.49 ml,0.025 mole) and ClCH2COCl (1.99 ml,0.025 mole), the color (pal Brown), 57%, m.p. (Oily), T.L.C. (MET:TOL) (3:2) (Rf, 0.45).
I.R spectra (C=O) str. (1734)cm-1, (C=C) str. Aromatic (1587) cm-1, (N-H) str. Sulfone (3317)cm-1, AZO (1438-1475) cm-1, (C-H) str. Aliphatic (2937-2976) cm-1, (C-Cl) 572 cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6, 400 MHz), (N-CH-C) (d2.379), (CO-CH-Cl) (d.2.734-2.776), (3H,OCH3) (s,3.282),(Ar-H) (6.366-6.649), (HC=N) pyrimidine (6853-7.545), (OH) (s 9.153), (N-H) Sulfone (10.761), DMSO(2.5).
C13-NMR-spectrum, (δ ppm), (DMSO-d6, MHz), 27.15 ,64.42, 83.79, 113.13,124.05, 131, 136, 152.81,153, 158.51, 170.54.
4-(2-(4-Bromo phenyl)-3-chloro-4-oxoazitidine-1-yl)-N-(pyrimidine-2-yl)-3-(pyrimidine-2-yldiazenyl)benzenesulfonamide[L4]
Was prepared from shiff base [B4] (5.229 gm, 0.01 mole), Et3 N (3.49 ml,0.025 mole) and ClCH2COCl (1.99 ml,0.025 mole), the color (yallow), m.p. (55-57) °C , T.L.C. (MET:TOL) (3:2) (Rf, 0.86).
I.R spectra (C=O) str. (S.1743)cm-1, (C=N) Pyrimidine (1627)cm-1, (C=C) str. Aromatic (1598) cm-1, (N-H) str. Sulfone (3417)cm-1, AZO(1463) cm-1, (C-H) str. Aliphatic(2922-2983) cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz) ), (N-CH-C) (d 3.543), (CO-CH-Cl) ( d.4.992), (Ar-H) (m 7.122-7.677), (HC=N) pyrimidine (m 7.820-8.643), (N-H) Sulfone(s 11.166), DMSO (2.5).
4-(3-chloro-2-(4-(nitro phenyl)-4-oxoazitidine-1-yl) -N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl)benzenesulfonamide[L5]
Was prepared from shiff base [B5] (4.89gm, 0.01mole), Et3 N (3.49 ml,0.025 mole) and ClCH2COCl (1.99 ml,0.025 mole), the color (Green yellow), m.p. (123-125)°C,T.L.C. (MET:TOL) (1:4 ) (Rf, 0.45).
I.R spectra (C=O) str. (1735)cm-1, (C=N) Pyrimidine (1678)cm-1, (C=C) str.Aromatic (1585) cm-1, (N-H) str. Sulfone (3392)cm-1, AZO (1442-1465) cm-1, (C-H) str. Aliphatic (2850-2920) cm-1.
1HNMR spectrum, (δ ppm), (DMSO-d6 MHz) ),(N-CH-C) (d. 5. 350),(CO-CH-Cl) ( d.5.798), (Ar-H) (m 6.675-7.820), (HC=N) pyrimidine (m 8.178-9.162), (N-H) Sulfone (s 11.143) ,DMSO(2.5).
C13-NMR-spectrum, (δ ppm) ,(DMSO-d6 ,MHz),112.569 ,115.351, 118.775, 125.624, 129.262,134.612, 137.822, 143.601,147.025, 150.449,158.795,166.072,169.924,187.687.
Results And Dicussion
Sulphaziazine was chosen as a starting material for the synthesis of new Azo comp.(S1) through a diazo-coupling reactionwith (2-amino pyrimidine) scheme[ 1]. the appearance of stretching band of primary aromatic amine (NH2) at (3427-3358)cm-1and Azo group (N=N) at (1440-1409)cm-1,and showed-1H-NMR spectrum of the compound characteristic signals:( 6.015, 2H, -NH2), (11.294 ,1H, NH-Salfone)are attributed to the formation of Azo compound.
The condensation reaction of Azo comp.with different aromatic aldehydes in the presence of glacial Acetic acid as solvent Scheme[2]gave the corresponding Schiff bases derivatives (B1-B5). The disappearance of the absorption bands at(3427-3358)cm-1 (-NH2) and appearance absorption bands of( C=N )Imine at (1639-1680 interaction with C=N pyrimidine rings) cm-1 were confirm the structures of the compounds (B1-B5) 1H.NMR spectrum of these Schiff bases showed the signal of imine proton at about (8.5-8.8ppm).
Schiff bases (B1-B5) that reacted with chloro acetyl chloride in the presence of tri ethyl amine in dry dioxane by a [2+2] cyclo addition reaction Scheme[3] to give corresponding β-lactam derivatives (L1-L5)[15]. appearance of the absorption bands of carbonyl lactam ring at(1734-1743) ) cm-1, these stretching frequencies in monocyclic lactams indicates that the carbonyl group in β-lactam behaves like” ester” group[16] . The spectra 13C-NMR of carbonyl Lactam showed the typical resonance at δ (169-187) ppm. the values outside this range are possible if strong electron donating or electron withdrawing groups are present on the adjacent carbon atoms. example for this, the spectrum 13CNMR of( L1, L3) showed the carbonyl carbon signal at (169.71, 170.54)respectively. whereas the carbonyl group in (L5) resonated at (187.687)ppm[17].
The mecanisim of mono-β-lactam formation via [2+2] cyclo addition showen in the Scheme(4)[18].
![Scheme 4: mecanisim of [2+2] cyclo addition Biological part 2-Azetidinone Derivative as anticancer](http://www.orientjchem.org/wp-content/uploads/2018/01/Vol34No1_Syn_Rad_sch4-150x150.jpg) |
Scheme 4: mecanisim of [2+2] cyclo addition Biological part 2-Azetidinone Derivative as anticancer
|
Materials and Methods
Methyl thiazolyl tetrazolium (MTT) Solution
Methyl thiazolyl tetrazolium (0.2g) (Bio-world, USA) was dissolved in 100 ml of PBS in order to prepare a 2 mg/ml concentration of the dye. The solution was filtered through 0.2 µm syringe filter to remove any blue formazan product, and then stored in sterile, dark, screw-capped bottles at 4°C. The solution was used within no longer than 2 weeks of preparation.
Cytotoxicity assay [22,23]
MTT cell viability assay was conducted on 96-well plates (Santacruz Biotechnology, USA), MCF7 Human Breast cancer cells were seeded at 10000 cells/well, 200 μl of cells in growth medium were added to each well of a sterile 96-well microtitration plate. The plates were sealed with a self-adhesive film, lid placed on and incubated at 37°C.
After 24hr or confluent monolayer is achieved, when the cells were in exponential growth, the medium was removed and serial dilutions of the extract were added to the wells at 2-fold serial dilutions (dilution the stock1/10ml). triplicates were used for each. Control cells treated with Serum Free Media only. Afterwards, the plates were re-incubated at 37°C for 72 hrs.
Cell viability was measured after 72 hrs of exposure by removing the medium, adding 28 µl of 2 mg/ml solution of MTT (Bio-World, USA) and incubating for 1.5h at 37°C. After removing the MTT solution, the crystals remaining in the wells were solubilised by the addition of 130 µl of DMSO (Dimethyl Sulphoxide) (Santacruz Biotechnology, USA) followed by 37°C incubation for 15 min with shaking.
The absorbency was determined on a microplate reader (Biochrom, UK) at 584 nm (test wavelength); the assay was performed in 5 replicates.
Endpoint parameters that are calculated for each individual cell line included:
1-Percentage of cell growth or percentage of cell proliferation (PR) = mean of treatment / mean of control.
2-The lowest concentration that kills 50% of cells (LC50).
Cell culture for Normal embrynoic cells
The rat embryo fibroblast (REF) cell line were cultured in a RPMI-1640 medium with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin and then incubated at 37 °C. All the cell lines were supplied by theIraqi Biotechnology Company, Cell culture lab, Baghdad, Iraq. These cells are regularly assessed for standard growth characteristics, and they are regularly authenticated.
The anticancer activity of the synthesized derivative(L1) in vitro was determined against an human breast MCF-7 cancer cell line using MTT cell viability assay . In general the result of the synthesized compound showed better anticancer potential (IC50 = 2.900µM) after 72 hr.
In figure (33)shows that the toxicity effect for [ 4-(3-chloro-2-(4-(di methyl amino)phenyl)-4-oxoazitidine-1-yl) -N-(pyrimidine-2-yl)-3-( pyrimidine-2-yldiazenyl)benzene sulfonamide][L1], which increase with increase the concentration.
In figure (34)shows the half lethal concentration which equal (LC50 2.9 µM) which kill the half number of cell and it will be absolutely lethal with increase concentration.but (LC50 ) for normal embrynoic cells equal (9.59 µM) as shown in figure (36) This means that the derivative is likely to be a killer of the cancer cell without affecting the normal cells when using its lethal concentration for half cells.
Table 1: Cell viability Values
|
Conc. (uM) |
0.07 |
0.155 |
0.31 |
0.625 |
1.25 |
2.5 |
5 |
10 |
|
Cell viability |
78.1 |
74.33 |
68.755 |
73.2 |
66.34 |
61.28 |
50.08 |
50 |
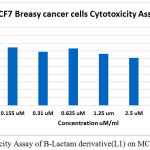 |
Figure 1: Cytotoxicity Assay of B-Lactam derivative(L1) on MCF7 Breasy cancer Click here to View figure |
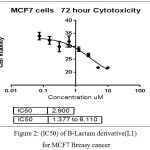 |
Figure 2: (lC50) of B-Lactam derivative(L1) forMCF7 Breasy cancer Click here to View figure |
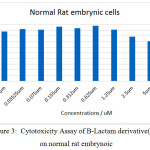 |
Figure 3: Cytotoxicity Assay of B-Lactam derivative(L1) on normal rat embrynoic Click here to View figure |
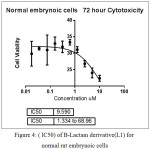 |
Figure 4: (lC50) of B-Lactam derivative(L1) for normal rat embrynoic cells Click here to View figure |
 |
Figure 5: 20X MCF7 cell control Click here to View figure |
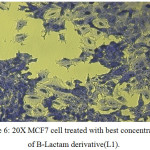 |
Figure 6: 20X MCF7 cell treated with best concentration of B-Lactam derivative(L1). Click here to View figure |
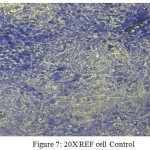 |
Figure 7: 20X REF cell Control Click here to View figure |
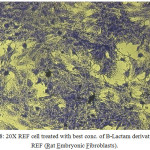 |
Figure 8: 20X REF cell treated with best conc. of B-Lactam derivative (L1) REF (Rat Embryonic Fibroblasts). Click here to View figure |
Conclusion
In this study we are reported synthesis of many B-Lactam derivatives via Staudinger Reaction [2+2] cyclo addition .The work included preparation of Azo-Schiff base compounds from sulphadiazine as the first step then cyclization process for these compounds by using chloro acetyl chloride in the basic medium and low temp. (0-10)οC These derivatives were found to be stable at room temperature and some of them Possess strength oily or sticky. These derivatives confirmed from spectral data analysis; FT-IR, H1 NMR and C13 NMR.
Acknowledgment
The authors are thankful to assist. prof. Dr. Ahlam Kadhum Naeem Al-yasseen, College of education for girls-Biology, Kufa University andIraqi Center for Cancer and Medical Genetic Research, Baghdad, Iraq. Also we are really appreciate the help of Mr.Abbas almulla.
References
- Salim Mohammed, A. ; Zebary, H. Y. S.;” Spectrophotometric Determination of Sulfadiazine via Diazotization and Coupling Reaction – Application to Pharmaceutical Preparations”, Raf. J. Sci ,2013,Vol. 24: No.6, pp. 61-73.
- Who Model List of Essential Medicines; ” World Health Organization. October 2013, Retrieved 22 April 2014.
- Ugras, H.I.; Basaran,I.; Kilic ,T. ; Cakir, U. J. Heterocyclic Chem., 2006, 43 : 1679 .
CrossRef - Chatterjee, S. ; Bhattacharyya,S.; ” Schiff Bases As a Source of Potent Molecules with Anti-Cancer Potential: A Short Review”,Asian Journal of Biochemical and Pharmaceutical Research,2015,Vol. 5, 4 ,pp.86-97.
- Al-Assadi , K, M.J.;” Synthesis and Characterization of Ni2+ and Cu2+ Schiff-base Complexes and Their Study for Electrical Properties”, Journal of Basrah Researches ((Sciences)),2011 :V. 37,No. 3 A.
- Sonnekar, VS. ; Jadhav , W.N. ; Dake, SA. ; Pawar, RP. .;” Synthesis and Antimicrobial and antifungal activities of novel Bis-imine Derivatives”, Research Journal of Pharmaceutical, Biological and Chemical Sciences(RJPBCS), 2013,Vol. 4 ,Issue 2 Page No. 1411-1418.
- Mohamed ,S. S.; Al-bashier, S. M.; Shalfoh, E. S. ; Fhid ,O.;” Microwave Assisted one-pot Synthesis and Screening of some schiff’s bases of Sulfanilamide”,Journal of Chemical and Pharmaceutical Research, 2012,4(5):2512-2516.
- Gharamaleki, J. A.; Akbari, F.; Karbalaei, A.; Ghiassi , K. B. ; Olmstead, M. M. ;”Synthesis, Characterization and Crystal Structure of a New Schiff Base Ligand from a Bis(Thiazoline) Template and Hydrolytic Cleavage of the Imine Bond Induced by a Co(II) Cation”,Open Journal of Inorganic Chemistry, 2016, 6, 76-88.
CrossRef - van der, F. S. ; Koten ,G. v. ;” Syntheses of 3-Amino-2-azetidinones: A Literature Survey”,Tetrahidron ,1991, Vol. 41. No 36,Pp. 7503-7524 .
- Gilchrist, T.,” Heterocyclic Chemistry“. Harlow: Longman Scientific (1987).
- Ehmann, D. E.; et al. ;”Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor”. PNAS. ,2012,109 (29), 11663–11668.
CrossRef - Tidwell , Thomas T.; “Hugo (Ugo) Schiff, Schiff Bases, and a Century of β-Lactam Synthesis”. Angewandte Chemie International Edition, 2008,47 (6): 1016–20.
CrossRef - Staudinger ,H. ; “Zur Kenntniss der Ketene. Diphenylketen”. Justus Liebigs Ann. Chem., 1907,356 (1-2): 51–123.
CrossRef
- Nangia, A.; Biradha, K.; Desiraju, G.R.; “Correlation of biological activity in β-lactam antibiotics with Woodward and Cohen structural parameters: A Cambridge database study”. J Chem Soc, Perkin Trans., 1996,2 (5): 943–53.
CrossRef
- Naser, A. W. ; Majeed ,A. M.; “Synthesis of some new sulfonamide derivatives based on 1,3,4-oxadiazole”., Journal of Chemical and Pharmaceutical Research, 2015,7(3):300-306.
- Anusha, K. ; Pradeep Kumar, Y.; Vara Prasad, M. ; raju V. B. m. ; Gopinath ,C.;”A REVIEW ON 2-AZETEDINONES”, Journal of Global Trends in Pharmaceutical Sciences( JGTPS), 2015 ,6 (1), (12388 – 2402).
- Magtoof, M . S. ; Hassan, Z. S.;”Synthesis and Characterization Of Some 3-Phenylthio/3-Phenoxyazetidine-2- One: Application of Two Dimensional NMR HMQC 1H-13C, Cosy 1H–1H And Mass Spectroscopy” National Journal of Chemistry(NJC), 2011,Vol. 41, 90- 105 .
- Parmar ,K. ; Modi, V. ; Prajapati, S. ; Patel, R., ;”A Facile And Expeditious Approach For The Synthesis Of 2-Azetidinone Derivatives With Microbial Activity”.,Asian Journal of Biochemical and Pharmaceutical Research, 2011,Issue 2 (Vol. 1) ISSN: 2231-2560 .
- yaqoob, R. H. ; Fahad, T.A. ;” Preparation, Characterization and Antimicrobial Activity of New Azo Complexes Containing Paracetamol Moiety ” , J of world of pharmaceutical Research , 2015,5,01,35-43 .
- Abbass. A. F. ; Zimam ,E. H.; “Synthesis , characterization and study biological activity of some new pyrimidine and 1 , 2 , 3 , 4-tetrazole derivatives based on sulfadiazine”, International Journal of ChemTech Research 2016,vol. 9, no. 11, pp. 206–217.
- Mohammed ,H. ; Zimam, E. H. ,Journal of Kufa for Chemical Science, 2012,Vol. No.6,pp 130-141.
- Freshney,R.I., of animal cells: A manual for basic technique,5th ed., John Wily & sons, Ianc. publication, New York, (2005).
CrossRef - Al-Shammari, A. M.; Salman, M. I.; Saihoodm Y. D.; Yaseen ,N. Y.; Raed K.; Shaker, H. K.; Ahmed, A.; Khalid, A. ; Duiach, A.,” In Vitro Synergistic Enhancement of Newcastle Disease Virus to 5-Fluorouracil Cytotoxicity against Tumor Cells”Biomedicines, 2016,4, 3.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









