Synthesis and Characterization of Block Copolymers of Polystyrene and Thermotropic Liquid Crystalline Polyesters
Cheng-Chih Chen1 and Chih-Hung Lin2
1Department of Cosmetic Science, Chang Gung University of Science and Technology, No.261, Wenhua 1st Rd., Guishan Township, Taoyuan County 33303, Taiwan, ROC.
2Center For General Education, Chang Gung University of Science and Technology, No.261, Wenhua 1st Rd., Guishan Township, Taoyuan County 33303, Taiwan, ROC.
Corresponding Author E-mail: chlin@mail.cgust.edu.t
DOI : http://dx.doi.org/10.13005/ojc/340114
A series of block copolymers containing a thermotropic liquid crystalline polymer and an amorphous polystyrene have been synthesized. The block copolymers were prepared by solution polycondensation of α,ω-dicarboxylic acid-terminated polystyrene with 4,4’-dicarboxy-1,6-diphenoxy- hexane and 4,4’-bis(2-hydroxyalkyloxy)biphenyls in a mixture of triphenylphosphine, hexachloroethane, and pyridine. The obtained polymers were fractionated to isolate the pure block copolymer. The block copolymers were characterized by FT-IR spectroscopy, differential scanning calorimetry, thermogravimetric analysis, optical polarizing microscopy measurement. Optical polarizing microscopy measurements verify the presence of a smectic liquid crystalline phase for the block copolymers. The block copolymers represent a microphase-separated morphology, i.e., both amorphous and liquid crystalline properties were displayed by the polystyrene and liquid crystalline polyester segments respectively.
KEYWORDS:Block Copolymer; Polystyrene; Thermotropic Liquid Crystal; Microphase
Download this article as:| Copy the following to cite this article: Chen C. C, Lin C. H. Synthesis and Characterization of Block Copolymers of Polystyrene and Thermotropic Liquid Crystalline Polyesters. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Chen C. C, Lin C. H. Synthesis and Characterization of Block Copolymers of Polystyrene and Thermotropic Liquid Crystalline Polyesters. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=43181 |
Introduction
In recent years, the polymers has attracted increasing interest their have a microphase separations systems. Microphase separations of polymer is good material forsolar cells. [1-13]. Such polymers included block or graft copolymers comprised of amorphous/ main-chain liquid crystalline [9-13] and amorphous/side-chain liquid crystalline blocks[3-8]. Adams and Gronski synthesized the synthesis of block copolymers containing a side-chain LCP and polystyrene [4]. Gottschalk and Schmidt prepared graft copolymers containing a side-chain LCP and polystyrene [3]. Chiellini et al. reported the ABA block copolymers containing a main-chain LCP B block and polystyrene A block [13].
In this research, LCP and block copolymers of polystyrene were prepared by solution polycondensation of α,ω-dicarboxylic acid-terminated polystyrene with 4,4’-dicarboxy-1,6-diphenoxyhexane and 4,4’-bis(2-hydroxyethyloxy)biphenyls in a mixture of triphenylphosphine, hexachloroethane, and pyridine. The block copolymers had a were well-separated and characterized by differential scanning calorimetry (DSC), optical polarizing microscopy (OPM), viscometer, FT-IR.
Material and Methods
Proton nuclear magnetic resonance (1H NMR) spectra (400 MHz) were recorded on a Bruker AM 400 spectrometer. FT-IR spectra were measured on a Nicolet 520 FT-IR spectrometer. Thermal transitions and thermodynamic parameters were determined using a Seiko SSC/5200 differential scanning calorimeter equipped with a liquid nitrogen cooling accessory. Heating and cooling rates were 10ºC/min. Thermal transition reports were collected during the second heating and cooling scans. Thermogravimetric analysis was performed on a Seiko TG/DTA 200 thermal analyzer. A Carl-Zees Axiphot polarized optical microscope equipped with a Mettle FP 82 hot stage and a FP 80 central processor were used to analyze the anisotropic textures. Gel permeation chromatography (GPC) was run on an Applied Biosystem 400 solvent delivery system equipped with UV and IR detectors.
Synthesis
The general synthetic routes of the intermediates and target molecule were shown in scheme 1. The purity and chemical structures of the intermediates and target compounds could be easily verified by TLC and 1H-NMR spectroscopy. The synthetic procedures and chemical analyses of each product were described sequentially below.
4,4’-Dicarboxy-1,6-diphenoxyhexane(1M)
Ethyl 4-Hydroxybenzoate (3.33g, 0.02mol) and 1,6-dibromohexane (2.44g, 0.01mol) and sodium carbonate (2.65g) were added to DMF(10mL). The solution was heated to 150°C for 4 hours then was cooled to room temperature. The solution slowly added to distilled water (1000mL), put in the refrigerator for at least 12 hours, and then filtered and dried. The crude product added potassium hydroxide (2.8g, 0.05mol) was dissolved in ethanol (50mL) and distilled water (10mL). The solution was refluxed for 4h then poured into distilled water (2000mL) heated to 60°C, added concentrated hydrochloric acid then put in the refrigerator for at least 12 hours, and then filtered and dried. The remaining white solid was recrystallized from DMF/ethanol to yield 2.69g(75%). 1H-NMR (CDCl3, δ, ppm): 1.57~1.62 (m, 4H, -CH2-CH2-CH2-), 1.84~1.92 (m, 4H, -O-CH2-CH2-CH2), 4.09~4.13 (t, 4H, -Ph-O-CH2-CH2-), 7.06~7.14 (d, 4H, aromatic protons), 7.88~8.04 (d, 4H, aromatic protons). 12.56~12.72 (t, 2H, -COOH).
4,4’-Bis(2-hydroxyethyloxy) biphenyl (2M)
4,4’-Bis(3-hydroxypropyloxy) biphenyl (3M)
4,4’-Bis(4-hydroxybutyloxy) biphenyl (4M)
4,4’-Bis(5-hydroxypentyloxy) biphenyl (5M)
4,4’-Bis(6-hydroxyhexyloxy) biphenyl (6M)
Compounds 2M~6M were prepared by a similar method. The synthesis of compound 6M is described below.
4,4’-Dihydroxydiphenyl (9.3 g, 0.05 mol) and 6-chloro-1-hexanol (15.1 g, 0.11 mol) were added to a solution of sodium hydroxide (4.4 g, 0.11 mol) in ethanol (200 mL, 95%). The solution was heated to reflux for 20 hours then was cooled and the solid salt was removed by filtration. The remaining solid was recrystallized from DMF/H2O to yield 15.6 g (81%) of crystals. 1H-NMR (CDCl3, δ, ppm): 1.37~1.44 (m, 12H, -CH2-CH2-CH2-), 1.68~1.76 (m, 4H, -Ph-O-CH2-CH2-), 3.36~3.40 (t, 4H, -CH2-OH), 3.95~3.99 (t, 4H, -Ph-O-CH2-), 4.35~4.38 (t, 2H, -OH), 6.95~6.98 (d, 4H, aromatic protons), 7.49~7.52 (d, 4H, aromatic protons).
Compound 2M was 65.3% yield.. 1H NMR (δ, CDCl3, TMS, 300 MHz): 3.72~3.74 (t, 4H, -CH2-OH), 3.99~4.03 (t, 4H, -Ph-O-CH2-), 4.86~4.87 (t, 2H, -OH), 6.98~7.00 (d, 4H, aromatic protons), 7.51~7.54 (d, 4H, aromatic protons).
Compound 3M was 81.5% yield. 1H NMR (δ, CDCl3, TMS, 300 MHz): 1.87~1.89 (m, 4H, -CH2-CH2-CH2-), 3.56~3.58 (t, 4H, -CH2-OH), 4.04~4.08 (t, 4H, -Ph-O-CH2-), 4.55~4.56 (t, 2H, -OH), 6.96~6.99 (d, 4H, aromatic protons), 7.50~7.53 (d, 4H, aromatic protons).
Compound 4M was 78.2% yield. 1H NMR (δ, CDCl3, TMS, 300 MHz): 1.55~1.61 (m, 4H, -CH2-CH2-OH), 1.72~1.81 (m, 4H, -Ph-O-CH2-CH2-), 3.44~3.57 (t, 4H, -CH2-OH), 3.98~4.08 (t, 4H, -Ph-O-CH2-), 4.44~4.57 (t, 2H, -OH), 6.94~6.98 (d, 4H, aromatic protons), 7.47~7.53 (d, 4H, aromatic protons).
Compound 5M was 75.3% yield. 1H NMR (δ, CDCl3, TMS, 300 MHz): 1.45~1.46 (m, 8H, -CH2-CH2-CH2-), 1.71~1.76 (m, 4H, -Ph-O-CH2-CH2-), 3.38~3.42 (t, 4H, -CH2-OH), 3.96~4.02 (t, 4H, -Ph-O-CH2-), 4.36~4.40 (t, 2H, -OH), 6.95~6.98 (d, 4H, aromatic protons), 7.48~7.51 (d, 4H, aromatic protons).
Synthesis of α,ω-dicarboxylic acid-terminated polystyrene (PS precursor polymer) (PS)
Styrene (136 g, 1.31 mol) and 4,4’-azobis-4-cyanovaleric acid (3.41 g) were dissolved in 600 mL of toluene under nitrogen in a two-necked flask equipped with a reflux condenser. The solution was stirred at 90ºC for 10 hours. After cooling, the product was isolated by precipitation in methanol, filtered, washed with water, and dried at 70ºC under vacuum to yield PS polymer(90.8%). Molecule weight obtained from GPC was Mn = 26918 g/mol, Mw = 34724 g/mol, PDI = 1.29.
Synthesis of a liquid Crystalline Polymer (LCP2-6)
Polymers LCP2-6 were prepared with a similar method. The synthesis of LCP2 is described below. Triphenylphosphine (1.66 g, 6.32 mmol) and hexachloroethane (1.49 g, 6.32 mmol) were dissolved in 10.0 mL of pyridine under nitrogen atmosphere. Compound 1M (0.57 g, 1.59mmol) was added and the resulting solution was heated to 140ºC for 15mins. The solution was cooled to room temperature then added compound 2M (0.44 g, 1.59mmol), the solution was stirred at 120ºC for 18 hours. After cooling, the resulting solution was precipitated in 200 mL of methanol, filtered, and stirred 30 mins., respectively. The product was filtered and dried under vacuum to yield LCP2 (0.89 g, 91.2%).
Synthesis of PS Based liquid Crystalline Block Copolymers 1P-5P
The synthetic procedure of copolymer 1P-5P is shown in Scheme 1. The series copolymers synthesized from PS precursor polymer, compound 1M and compound 2M to 6M were marked as copolymer 1P-5P. This series reaction was reacted by PS precursor polymer and LCP block weight ratio 1:1. Copolymers 1P-5P were prepared with a similar method as the polyester copolymer 1P described below.
The synthesis of copolymer 1P is here described as an example. Triphenylphosphine (1.66 g, 6.32 mmol) and hexachloroethane (1.49 g, 6.32 mmol) were dissolved in 7.0 mL of pyridine under nitrogen atmosphere. PS precursor polymer (2.0 g) and compound 1M (1.13g, 3.17mmole) was then added and the resulting solution was heated to 140ºC for 15mins. The solution was cooled to room temperature then added compound 2M (0.87 g, 3.17 mmol). After the addition was completed, the solution was stirred at 120ºC for 18 hours. After cooling, the resulting copolymer was precipitated in 200 mL of methanol, filtered and dried, respectively. The product was dried at 70ºC under vacuum to yield copolymer 1P (3.61 g, 90.2%).
Preparation of Fractional PS Based liquid Crystalline block Copolymers (1a-5a, 1b-5b, and 1c-5c )
The copolymers 1P-5P was further fractionated according to the steps. First, the copolymer was stirred in 250 mL of THF at room temperature for 24 hours and then filtered. The dissolved polymer was isolated by reprecipition in methanol, then filtered, and dried to form copolymer as “fraction a”. Further, the undissolved part was stirred in 250 mL of chlorobenzene at 100ºC for 24 hours and filtered. The dissolved polymer was isolated by reprecipitation in methanol, and then dried to form copolymer as “fraction b”. The received solid was washed with acetone and methanol and then dried to form copolymer as “fraction c”. Table 1 summarizes the resulting ratios of these fractionated copolymers.
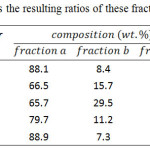 |
Table 1: Summarizes the resulting ratios of these fractionated copolymers Click here to View table |
Results and Discussions
In this study, we synthesized six compounds(1M-6M), five liquid crystalline polymers(LCP2-6), one PS precursor polymer and five copolymers(1P-5P). The molecular structure and the general synthetic procedures of these compounds were shown in Scheme 1, and all the final products were examined by the nuclear magnetic resonance spectrometer in order to verify the correction of the molecular structure. The thermal properties and liquid crystalline mesophase of compounds 2M-6M, LCP2-LCP6 and copolymer 1P-5P were measured using DSC and OPM.
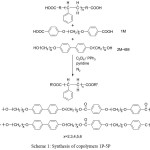 |
Scheme 1: Synthesis of copolymers 1P-5P Click here to View scheme |
The changes of phase transition temperature and enthalpy of compounds 2M-6M with five different length spacers were listed in Table 2. All the compounds 2M-6M exhibited an enantiotropic SB phase. Figure 1A presents the optical polarizing microphotography of 6M, showed SB phase texture at 165.9℃. Figure 2 depicts a DSC analysis diagram of compounds 2M-6M. According to the results presented by Table 2 and Figure 2, compounds 2M-6M show an odd-even effect; the compounds with even number of alkyl spacer (2M, 4M, 6M) have wider liquid crystal phase temperature range than the compounds with the odd one (3M, 5M). Furthermore, the isotropic temperature of the compounds decreases when the length of the alkyl spacer increases.
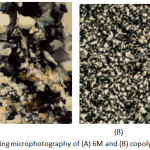 |
Figure 1: Polarized optical microphotography of (A) 6M and (B) copolymer 5b Click here to View figure |
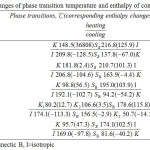 |
Table 2: The changes of phase transition temperature and enthalpy of compounds 2M-6M Click here to View figure |
The thermal analysis data and intrinsic viscosity (I.V.) values of liquid crystalline polymers (LCP2-6) are listed in Table 3. The Tg of LCP2-6 decreases with the increase of their alkyl spacer length. Additionally, the Tm of LCP2-6 also exhibits an odd-even effect. The I.V. values of LCP2-6 are between 0.066~0.183(dL/g); it increased with the increase of alkyl chain number of the spacer, indicating that longer soft chain compound has a better solubility.
Table 3: Characterization of liquid crystalline polymer (LCP2-6).
| polymer | Tg(ºC) | Tm(ºC) | ΔHm (mJ/mg) | ΔHi(mJ/mg) | Tda(ºC) | wt. loss(%) | I.V.b(dL/g) |
| LCP2 | 55.6 | 136.8 | – | 15.00 | 395.4 | 80.00 | 0.067 |
| LCP3 | 53.6 | 208.2 | – | 73.60 | 382.3 | 73.22 | 0.066 |
| LCP4 | 48.7 | 159.0 | 0.5 | 31.90 | 378.5 | 87.50 | 0.127 |
| LCP5 | 33.3 | 180.6 | 48.65 | 5.95 | 384.6 | 90.10 | 0.183 |
| LCP6 | 29.6 | 156.1 | 35.01 | 35.33 | 392.4 | 90.20 | 0.123 |
aTd is the decomposition temperature obtained by TGA
bIntrinsic viscosity (I.V.) is obtained by dissolution of block liquid crystalline polymer in 4-chlorophenol/1,1,2,2-tetrachloroethane(20/80% by weight) at 70ºC
Scheme 1 showed the synthetic path for the preparation of PS based liquid crystalline block copolymers. The polymer of the carboxylic acid-terminated PS precursor was prepared by a free radical polymerization of styrene using 4,4’-azobis(4-cyanovaleric acid) as initiator based on a procedure reported in the literature[18] The number average molecular weight of the PS precursor polymer was 2.7× 104 g/mol. The Polymer was determined by NMR end group analysis and titration method. Its functionality was 1.8. This result means that the coupling predominately occurred in the termination step.
The PS precursor polymer was subjected to solution condensation with 1M and 2M-6M in pyridine using triphenylphosphine dichloride as a condensation agent [14,15]. In this study, used solution condensation method.The 1M and 2M-6M were added dropwise to the solution of PS precursor polymer in pyridine in the presence of triphenylphosphine dichloride. Since there was a larger amount of PS precursor polymer than 1M and 2M-6M at the beginning of the reaction, it provided more opportunity to form the block copolymers of PS and LCP.
Table 1 summarizes the results of the copolymerization by condensation. The obtained copolymers were fractionated into three fractions: 1a-5a were THF soluble fraction, 1b-5b were chlorobenzene soluble fraction, and 1c-5c were chlorobenzene insoluble fraction. Figure 3 showed the PS precursor polymer, block copolymers 2a, 2b, 2c, and LCP2 of FT-IR spectra. The PS precursor polymer and LCP2 reveal the following; the special absorption bands at 2923 cm-1 and 2850 cm-1 are attributed to the -CH2 stretching absorption of the PS precursor polymer. The absorption band at 1736 cm-1 could be due to the C=O stretching absorption in the spectrum of LCP. This special absorption can be used to estimate the ratio of PS polymer block and LCP block in the resulting copolymer. In order to understand the nature of the copolymer produced, the THF soluble fractions, copolymer 1a-5a, were characterized by GPC, IR, DSC, and TGA. The GPC, DSC, and TGA results were summarized in Table 4. GPC obtained the relative molecular weights. This results demonstrate that a small amount of chain extensions from PS core occurred in these block copolymers. Moreover, the Tg and decomposition temperature (Td) values of 1a-5a were very close to PS precursor polymer. Figures 3A and 3B showed the IR spectra of PS precursor polymer and copolymer 2a, respectively. By the observation of C=O stretching absorption band(1736 cm-1), It’s worth noting that only a small number of LCP segments were present in the copolymer 2a. All these results explain that the THF soluble fractions(copolymer 1a-5a) belong to the PS precursor polymer extended with a short LCP segments.
Further, we selected chlorobenzene as the host solvent. Copolymers of THF-insoluble were further fractionated into chlorobenzene soluble fractions 1b-5b and insoluble fractions 1c-5c. Figure 3D showed 2c of the IR spectrum inferred that the chlorobenzene insoluble fraction contained predominately the LCP2 polymers (Figure 3E). The chlorobenzene soluble fractions 1b-5b seemed to be the block copolymers of PS and LCP. Figure 3C showed the IR spectrum of 2b, and both characteristics of PS and LCP block were presented in this spectrum.
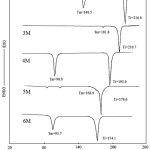 |
Figure 2: DSC analysis diagram of compounds 2M-6M Click here to View figure |
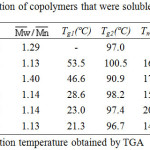 |
Table 4: Characterization of copolymers that were soluble in THF(1a-5a). Click here to View figure |
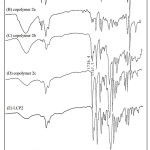 |
Figure 3: FT-IR spectra of (A) PS, (B) copolymer 2a, (C) copolymer 2b, (D) copolymer 2c, (E) LCP2 Click here to View figure |
Table 5: Characterization of copolymers copolymers that were soluble in chlorobenzene at 100°C(1b-5b).
| polymer | Tg1(ºC) | Tg2(ºC) | Tm(ºC) | ΔH (mJ/mg) | Tda(ºC) | wt. loss(%) | I.V.b(dL/g) |
| PS | – | 97.0 | – | – | 363.3 | – | 0.14 |
| 1b | – | – | 137.7 | 7.5 | 392.4 | 74.2 | 0.18 |
| 2b | 51.0 | 101.7 | 222.1 | 40.3 | 376.7 | 77.2 | 0.18 |
| 3b | 34.1 | 91.4 | 174.4 | 33.5 | 389.0 | 67.4 | 0.19 |
| 4b | 31.9 | 91.3 | 146.8 | 19.2 | 384.5 | 66.5 | 0.22 |
| 5b | 27.4 | 85.4 | 135.2 | 15.7 | 383.3 | 74.2 | 0.22 |
The thermal analysis data of 1b-5b and their intrinsic viscosity (I.V.) values, which were measured by dissolving the block copolymers in 4-chlorophenol/1,1,2,2-tetrachloroethane(20/80% by weight) at 70ºC, are summarized in Table 5. Their glass transition, which appeared at about 100ºC, could be attributed to the block movements from PS segments; and the melting endothermic peak presented at about 135~220ºC, can be referred to the crystalline-to-sematic phase transition of LCP2-6 block. Both transitions were very close to that of PS precursor polymer and LCP homopolymer. These results clearly indicated a microphase-separated morphology for the block copolymers. Figure 1B showed the optical polarizing microphotography of copolymer 5b, displayed a smectic phase texture at 92.2℃. The texture illustrates the bright region belonged to liquid crystal segment, and the dark region belonged to the PS segment. Basically, the LC domains were dispersed in the polystyrene matrix. Thermogravimetric analyses of 1b-5b reveal that all these block copolymers were thermally stable up to 380ºC. The I.V. values of 1b-5b were larger than those of the PS precursor polymer and LCP polymer. This indicates that the true block copolymers were found.
Conclusions
PS and LCP Block copolymers were prepared by solution condensation of telechelic PS with 4,4’-dicarboxy-1,6-diphenoxyhexane and 4,4’-bis(2-hydroxyethyloxy) biphenyls monomers. The received products were fractionated to isolate the most appropriated block copolymers. The IR spectrum of 1b-5b showed the -CH2 stretching absorption from PS precursor segment and the C=O stretching absorption from LCP segment. The I.V. values of 1b-5b were larger than those of the PS precursor polymer and LCP polymer. These indicate that the true block copolymers were found. The block copolymers reveal a microphase-separated morphology, i.e., both amorphous by the PS and LCP block respectively. Optical microscopy verified the presence of a smectic liquid crystalline phase. As a result, the block copolymers have potential applications in the field of polymer blends.
Acknowledgments
Research was supported in part by Chang Gung University of Science and Technology grant EZRPF3G0171、EZRPF3G0181.
References
- Nagahama, K.; Ueda, Y.; Ouchi, T.; Ohya, Y. Biomacromolecules. 2007, 8, 3938–3943
CrossRef - Nagano, S.; Matsushita, Y.; Ohnuma, Y.; Shinma, S.; Seki, T. Langmuir, 2006, 22; 5233-5236
CrossRef - Gottschalk, A.; Schmidt, H. W. Liq. Cryst. 1989, 5; 1619-1625
- Adams, J.; Gronski, W. Makromol. Chem., Rapid Commun. 1989, 10; 533-537
CrossRef - Hefft, M.; Springer, J. Makromol. Chem., Rapid Commun. 1990, 11; 397-401
CrossRef - Zaschke, B.; Frand, W.; Fischer, H.; Schmutzler, K.;, Arnold, M. Polym. Bull. 1991, 27;1-7
CrossRef - Galli, G.; Chiellini, E.; Yagci, Y.; Serhatli, E. I.; Laus, M.; Begnoozzi, M. C.; Angeloni, A. S. Makromol. Chem., Rapid Commun. 1993, 14; 185-191
CrossRef - Gottschalk, A.; Schmidt, H. W. Polym. Prepri. 1993, 34; 188-192
- Auman, B. C.; Percec, V. Polymer, 1988, 29; 983-990
CrossRef - Heitz, W. Macromol. Chem., Macromol. Symp. 1989, 26; 1-6
CrossRef - Sato, M.; Kobayashi, T.; Komatsu, F.; Takeno, N. Macromol. Chem., Rapid Commun. 1991, 12; 269-274
CrossRef - Hsu, C. S.; Chen, L. B. Mater. Chem. Phys. 1993, 34; 28-35
CrossRef - Chiellini, E. ; Glli, G. ; Angeloni, A. S. ; Bignozzi, M. C. ; Laus, M. ; Sergatli, E. I. ; Yagci, Y. Polym. Prepr. 1993, 34; 190-195
- Riess, W.; Heitz, W. US Pat. 1988, 4, 769, 423
- Eckharat, V.; Dicke, H. R.; Bottenbruch, L. Ger. Offen. 1988, DE 3, 623, 319
- Kiss, G. Polym. Eng. Sci.=1987, 27; 410-416
CrossRef - Viswanathan, R.; Johnson, B. C.; McGrath, J. E. Polymer. 1984, 25; 1827-1833
CrossRef - Lin, C. H. Des. Monomers Polym. 2013, 16, 537-542
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









