Synthesis and Characterization Benzimidazole Ring by using O-Phenylinediamine with Different Compounds and using Mannich Reaction to Preparation some of Derivatives
Chemistry Department, College of Education, University of Al-Qadisiyah , Iraq 2016-2017.
Corresponding Author E-mail: Abdullah.Kadhim@qu.edu.iq
DOI : http://dx.doi.org/10.13005/ojc/340152
The research includes synthesis and characterization Benzimidazole rings by using different compounds such as Urea, Thiourea and Carboxylic acid by reactant with (ο-phenylinediamine), then substitution hydrogen atom with present on nitrogen atom by reactant with primary and secondary amines according to Mannich reaction. Compounds was organized by using F.T.I.R and HNMR spectroscopy.
KEYWORDS:Benzimidazole; o-phenylinediamine; Thiouoria; Amines; Mannich Reaction
Download this article as:| Copy the following to cite this article: Kadhim A. J. Synthesis and Characterization Benzimidazole Ring by using O-Phenylinediamine with Different Compounds and using Mannich Reaction to Preparation some of Derivatives. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Kadhim A. J. Synthesis and Characterization Benzimidazole Ring by using O-Phenylinediamine with Different Compounds and using Mannich Reaction to Preparation some of Derivatives. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41709 |
Introduction
Benzimidazole is heterocyclic compound found in many natural and non-natura1 products, such as some vitamins(1).Therefore, benzimidazole substitutes tooks the attention of differents research groups, especially as compensation or replacement in the position 1,2 is very important sites in the impact of drug effective(2). In This study reported some of the ways to prepare benzimidazole 2-substitution, for the importance of this compound in the field of antibiotics,such as cancer,angiotensin-II receptor antegonests and antimicrobial properties(3)antifungal, antiparkinson,…etc(4).The NH group compounds are able to entering into N-alkylation and N-acylation according to Mannich and Michael reaction as in isatin compounds(5), which are similar with benzimidazoles. Mannich reaction very important reaction by converting some of the prepared compounds into other compounds are more important than in the biological field,(6).
Materials and Methods
Melting points were determined by using Melting PointSMP3 apparatus.
F.T.I.R. spectra were recorded by using Fourier Transform Infrared Spectrophotometer (F.T.I.R) 8400 S Shimadzu apparatus.
NMR Spectrometer 400 MHz, Avance III 400 Bruker, Germany.
Experimental Section
Synthesis 1,3-dihydro-benzimidazol-2-subsititution (A and B)
A mixture of o-phenylenediamine with urea to yield (A) and with thiourea to yield (B) in existence of HCl in equal concentrations was heated at 130c0 under reflux in alcohol solution until the evolution of ammonia ceased,(7).
Synthesis 2-Phenol-1H-benzimidazol (C)
A mixture of equal concentration (0.01mol) of o-phenylenediamine and Salicylic acid in 4N HCl (20 ml) was refluxed for 30 min. then cooled, filtered and recrystallized from absolute alcohol (3).
General Procedure for Synthesis of Compounds (A1) (B1) (C1)
A mixture of alcoholic solution with (A) , with (B) , with (C) (0.01 mol) and formaldehyde (15 ml, 40 %) was added slowly on alcoholic solution of (4-chloro-aniline ) (0.01 mol) the reaction mixture was stirred for three hours at room temperature and kept full night in the fridge. The solid obtained was filtered ,washed with cold ethanol, dried and crystallized form aqueous ethanol to give compounds (A1), (B1), (C1)(8).
General Procedure for the Synthesis of Compounds (A2) (B2) (C2)
A mixture equimolar of (A), (B), (C) with Diallylamine in presence formaldehyde were carried out 0-5CO by stirring with magnetic stirrer(9).
Table 1: show physical properties of compounds
|
Compound |
Molecular formula |
Solvent |
Yield % |
m.p. Cº |
Color |
|
A |
C7H6N2O |
Ethanol |
94 |
275-279 |
Yellow |
|
B |
C7H6N2S |
Ethanol |
66 |
114-118 |
Violet |
|
C |
C13H10N2O |
Ethanol |
72 |
155-161 |
Violet |
|
A1 |
C21H18N4OCl |
Ethanol |
63 |
Oil |
Yellow |
|
B1 |
C14H12N3ClS |
Ethanol |
62 |
Oil |
Nutty |
|
C1 |
C20H16N3ClO |
Ethanol |
64 |
Oil |
Nutty |
|
A2 |
C21H28N4O |
Ethanol |
70 |
Oil |
Yellow |
|
B2 |
C14H17N3S |
Ethanol |
73 |
Oil |
Nutty |
|
C2 |
C20H21N3O |
Ethanol |
76 |
Oil |
Nutty |
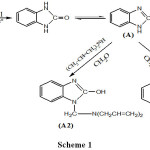 |
Scheme 1 |
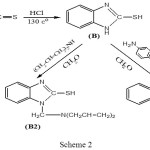 |
Scheme 2 |
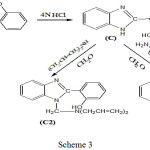 |
Scheme 3 Click here to View shceme |
Result and Discussion
The aim of the research is to synthesis mannich bases from different Benzimidazole rings with primary and secondary amines by using formaldehyde as catalyst.
1H-benzimidazole-2-ol (A)
Infrared spectroscopy of this compound showed a broadband at 3348-3433 cm-1 refers to O-H group, as well as the emergence of weak peak at 1739 cm-1 indicate that O-H group turn to C=O group by resonance between O-H and N atom , the spectroscopy showed too other peaks at 3132-3178cm-1 refers to N-H group and at 3024 cm-1 to C-H aromatic. Table (2) shows other peaks to this compound. 1HNMR (400 MHz , DMSO) δ(ppm) Ar(6.97 Hz 1H),OH(5.45 Hz 1H),NH(5.45 Hz 1H)
1-{[(4-chlorophenyl)amino]methyl}-1H-benzimidazol-2-ol (A1)
Infrared spectroscopy showed overlap of (O-H) peak with (N-H) peaks at 3261-3481 cm-1 after substitution (H) atom by compound (4-chloro aniline), also emergence absorption peak at 2921-2979 cm-1 refers to (C-H) aliphatic . Table (2) shows the other peaks for this compound. 1HNMR (400 MHz , DMSO) δ(ppm) Ar (7.09,7.10 Hz and 7.25) , OH(5.24Hz) , CH2(5.77-5.81Hz), NH(3.84-3.88Hz).
1-[(diallyl-amino)methyl]- 1H-benzimidazol-2-ol (A2)
Infrared measurements of this compound showed disappearance (N-H) peak duo to substitution H atom by compound (diallyl amine) and emergence new peak at 2908-2958 cm-1 refers to (C-H) and (CH=CH) respectively in addition to (O-H) group in 3355-3394 cm-1. Table (2) shows other peaks to this compound. 1HNMR (400 MHz , DMSO) δ(ppm) Ar (7.18,7.19 Hz and 7.32 Hz), OH(4.89 Hz), CH2 (4.76 Hz), CH=(5.87 Hz) , CH2 (3.41 Hz), =CH2(5.30 and 5.43 Hz).
1H-Benzimidazole-2-thiol (B)
Infrared spectrum for this compound showed absorption peaks sharp at 3379 cm-1 indicate to (N-H) group, also appearance absorption peak at 2360 cm-1 indicate to (S-H) group. Table (2) shows other absorption peaks for this compound. 1HNMR (400 MHz , DMSO) δ(ppm) Ar (broad 7.19-7.28Hz 2H) and (broad 7.64-7.73 Hz 2H),
1-{[(4-chlorophenyl)amino]methyl}-Benzimidazole-2-thiol (B1)
Infrared spectrum showed stay of absorption peaks of (N-H) group at 3224 cm-1 after substitution of (H) atom by compound (4-chloroaniline ) and emergence absorption peak at 2923 cm-1 indicate to (C-H) aliphatic. Table (2) shows other absorption peaks for this compound.
1-[(diprop-2-en-1-ylamino)methyl]-1H-benzimidazole-2-thiol (B2)
Infrared spectrum showed disappearance absorption peak of (N-H) after substitution of (H) atom by compound (Diallylamine) and appearance absorption peak at 2950 cm-1 indicated to (C-H) aliphatic. Table (2) shows other absorption peaks for this compound. 1HNMR (400 MHz , DMSO) δ(ppm) Ar(7.27,7.31 and 7.72 Hz ), SH(2.54 Hz 1H), CH2(4.70 2H), CH2-CH= (3.51-3.52 Hz 4H), CH= (5.07 Hz 2H) , =CH2(4.85-4.95Hz 4H).
2-(2-hydroxyphenyl)-1H-Benzimidazol (C)
Infrared spectrum showed broadband at 3402-3421cm-1 indicated to (OH) group and absorption peak at 3236 cm-1 indicated to (NH) group. Table (1) shows other absorption peaks for this compound. 1HNMR (400 MHz , DMSO) δ(ppm) Ar(7.23,7.24 and 7.77 Hz ) and (6.77, 6.89 and 7.05) , OH(6.74Hz ), NH(6.77Hz ).
2-(2-hydroxyphenyl)-1-{[(4-chlorophenyl)amino]methyl}-1H-Benzimidazol (C1)
Infrared spectrum showed appearance broadband at 3317-3417 cm-1 indicated to (OH) group and absorption peak at 3186 cm-1 indicated to (NH) group and new absorption peak at 2981cm-1 indicated to (C-H) aliphatic. Table (1) shows other absorption peaks for this compound. 1HNMR (400 MHz , DMSO) δ(ppm) Ar (7.03),(7.19-7.21) ,(7.76, 7.39) and (6.76, 6.86Hz) , OH(4.97Hz 1H), CH2 (5.49 Hz), NH(4.14 – 4.17Hz 1H).
2-(2-hydroxyphenyl)-1-[(diprop-2-enamino)methyl]-1H-Benzimidazol (C2)
Infrared spectrum showed survival of broadband for (OH) group at 3398 cm-1 and disappearance absorption peak of (N-H) for Benzimidazole ring duo to substitution hydrogen atom by formaldehyde and secondary amine and new absorption peaks at 2800-2977 cm-1 indicated to C-H aliphatic in propene . Table (1) shows other absorption peaks for this compound. 1HNMR (400 MHz , DMSO) δ(ppm) Ar(7.25-7.29Hz) and (7.32,7.44 , 7.70 , 7.79Hz ), OH(4.79Hz) , CH2(4.68Hz) , =CH(5.49-5.55Hz) , CH2-CH= (3.67-369Hz), =CH2(5.16-5.18 Hz).
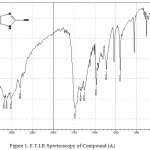 |
Figure 1: F.T.I.R Spectroscopy of Compound (A) |
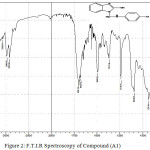 |
Figure 2: F.T.I.R Spectroscopy of Compound (A1) Click here to View figure |
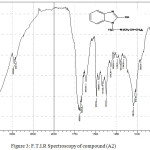 |
Figure 3: F.T.I.R Spectroscopy of compound (A2) Click here to View figure |
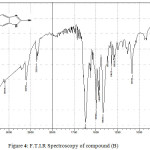 |
Figure 4: F.T.I.R Spectroscopy of compound (B) Click here to View figure |
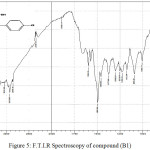 |
Figure 5: F.T.I.R Spectroscopy of compound (B1) Click here to View figure |
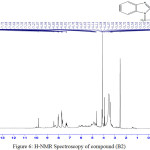 |
Figure 6: H-NMR Spectroscopy of compound (B2) Click here to View figure |
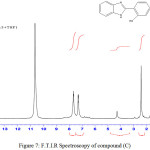 |
Figure 7: F.T.I.R Spectroscopy of compound (C) |
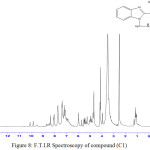 |
Figure 8: F.T.I.R Spectroscopy of compound (C1) Click here to View figure |
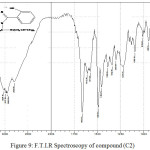 |
Figure 9: F.T.I.R Spectroscopy of compound (C2) |
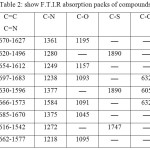 |
Table 2: show F.T.I.R absorption packs of compounds |
Acknowledgements
The author gratefully acknowledges Chemistry department, University of Qadissiah, Iraq for providing lab facilities to carry out this work.
Reference
- Ramanatham V., Sanjay D. V., Bobba. V. S. Kumar, Umesh N. B., Shekar B. B., and Uday C. Mashelkar. ARKIVOC 2008 (xiv) p 27-49.
- Shaimaa Adnan, Kasim Hassan and Hassan Thamer, Iraqi National Journal of Chemistry, 2014, volume53,66-75.
- Panneer Selvam, T. , P. P. Radhika, S. Janagaraj1, A. Siva Kumar, Research in Biotechnology, ISSN: 2229-791X 2011, 2(3):50-57.
- Mohan Babu Maradolla, Sunil Kumar Allam, Amaravathi Mandha, and G. V. P.Chandramouli, ARKIVOC 2008 (xv) 42-46.
- Khalaf Ahmed Jasim AL- Bayati, Tikrit Journal of Pure Science 17 (2) 2012 ISSN: 1813 – 1662.
- Suad M.Al-Araji and Rana A.Ali, Baghdad Science Journal Vol.9 (1) 2012.
- John B. Wright THF: Rsesarch Laboratories, The Upjohn Company, Kalamazoo, Michigan 1951,p 446 – 454.
- Badie A. Ahmed and Salem J. Mohammed. Iraqi National Journal of Chemistry, 2011 ,volume 44,582- 589.
- V. Ravichandran, S. Mohan, and K. Suresh Kumar. ARKIVOC 2007(xiv)51-57.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.










