Study of the Al, Cr, Fe and TI Impurities Coprecipitation on Coprecipitants of Various Natures in Potassium Dihydrogen Phosphate Solutions
Anna Zharova1,2 , Ilia Komendo2 and Roman Sandu3
, Ilia Komendo2 and Roman Sandu3
1D. Mendeleev University of Chemical Technology of Russia, Moscow, Russia.
2NRC «Kurchatov Institute» – IREA, Moscow, Russia.
3NRC «Kurchatov Institute», Moscow, Russia.
Corresponding Author E-mail: a.zharova.muctr@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340115
The main aim of this research is to obtain high purity potassium dihydrogen phosphate solution with Al, Cr, Fe and Ti impurity content < 0.05 ppmw (5∙10-6 wt.%) by the simple and effective process of impurities coprecipitation with insoluble precipitants. The completeness of the coprecipitation with different coprecipitants of the above-mentioned impurities in saturated potassium dihydrogen phosphate solutions (KH2PO4, KDP) was studied. Well-known coprecipitants such as inorganic phosphates, hydrated oxides and organic complexing agents were tried out to concentrate impurities in solid phase. Optimum conditions contributing to the maximum coprecipitation of the metal impurities were found for each coprecipitant. The obtained results are applicable both for KDP solution purification and for impurity concentration and separation in analytical chemistry.
KEYWORDS:KDP; Potassium Dihydrogen Phosphate; Coprecipitation; Inorganic Salts Purification
Download this article as:| Copy the following to cite this article: Zharova A, Komendo I, Sandu R. Study of the Al, Cr, Fe and TI Impurities Coprecipitation on Coprecipitants of Various Natures in Potassium Dihydrogen Phosphate Solutions. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Zharova A, Komendo I, Sandu R. Study of the Al, Cr, Fe and TI Impurities Coprecipitation on Coprecipitants of Various Natures in Potassium Dihydrogen Phosphate Solutions. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=43377 |
Introduction
Finding advanced method of producing high-purity potassium dihydrogen phosphate (KDP) – a raw material for rapid single crystal growth, constitutes a pressing objective due to the global implementation of projects relevant to developing laser units of ultra-high peak power, since single crystals of KDP have high laser strength (>5 GW/cm2) and wide range of optical transparence (200 ÷ 1500 nm), which makes them the most suitable material for manufacturing Q-switches and second-harmonic modulators1. Another advantage of potassium dihydrogen phosphate is possibility to grow large-scale single crystals. Rare earth- and thallium-doped KDP single crystals are also used as scintillating materials for detecting fast neutrons2,3.
In actual technology of the rapid growth of large-scale KDP single crystals raw materials with 3d-metal impurities concentrations below 0.1 ppmw per each metal are used. Requirements to the raw materials are so strict due to the fact that metal ions are strongly adsorbing on the prismatic faces of the growing crystal. Metal ions adsorption and their following incorporation into the crystal structure causes formation of crystalline defects and subsequently drastically changes both of crystalline perfection and growth rate of the crystal. It is well known that the metal ions the most affecting KDP single crystals’ growth rate and properties are Fe(III), Al(III), Cr(III) and Ti(IV) 4.
Early reported purification methods of inorganic salts of orthophosphoric acid in general describes effective removal of several impurities (usually one or two) which are not playing significant role in KDP single crystals grow processes, or residual content of the impurities not meet the requirements to the raw materials for rapid growth of single crystals. Present work is related to obtaining pure potassium dihydrogen phosphate solution with concentration of impurities which the presence the most unfavorable in KDP – Fe(III), Al(III), Cr(III) and Ti(IV) < 0.05 ppmw (5∙10-6 wt.%).
The effective and simple method of producing high-purity substances is the impurity coprecipitation with slightly soluble residues – coprecipitants.
The following compounds were used to study impurity’s coprecipitation processes in KDP solutions:
Phosphates of Al, Ca, Zr, since; these compounds are extremely slightly soluble5, so their precipitation makes it possible to adsorb impurities by the mechanism of isomorphous substitution;
Hydrated oxides of Al, Zr, Mn, since ; Al and Zr hydroxides are effective coprecipitants for Fe3+ and Cr3+ 6-9 ions, while hydrated manganese dioxide is known as a sorbing agent for some s- and d- elements (Mn2+, Ni2+, Co2+, Cu2+, Zn2+, Pb2+)10;
Macro-component – KDP. According to the relevant data11, Fe, Cr, Al and Ti impurities have positive distribution coefficients exceeding 1;
Organic precipitators (sodium N,N-diethyldithiocarbamate, cupferron), which form with metal ions Co, Fe, Mo, Ni, Ti and V slightly water-soluble compounds12.
Experimental
Apparatus and Materials
KDP impurity content analysis was carried out using photocolorimetric method similar to the relevant technique13 and atomic emission spectroscopy with inductively coupled plasma, using ICAP duo 6300 spectrometer (Thermo Scientific). Concentrations of impurities are presented in ppmw (parts per million by weight), 1 ppmw corresponds to 10-4% by weight.
The following reagents were used: potassium dihydrogen phosphate, phosphoric acid and potassium hydroxide with Al, Cr, Fe and Ti impurities content of <0.5 ppmw. Deionized water with its specific resistivity of ≥18.0 МOhм was used to prepare the solutions. PP labware was used to avoid the contamination by hardware material’s foreign impurities. pH measurements of the media were carried out using Ohaus Starter ST 2100 with ST 210 electrode.
The following salts were used to produce coprecipitant residues: aluminum nitrate, calcium nitrate, cupferron, manganese chloride, N,N-sodium diethyldithiocarbamate 3-aqueous and zirconyl nitrate. All reagents were pure grade.
Experimental Procedure
The micro-component coprecipitation were studied using reference solution of potassium dihydrogen phosphate, saturated at 20 °С, at the concentration of 220 g/L and its Al, Fe and Cr content increased to 5 ppmw (Table 1), by adding relevant metal’s salt solution. Adding titanium to the solution, accompanied by exceeding 0.7 ppmw, turned out to be impossible due to the slight solubility of titanium phosphate.
Table 1: Concentration of the metals in question in the reference KDP solution
|
Element |
Concentration, ppmw |
|
Al |
5.0 |
|
Cr |
5.0 |
|
Fe |
5.0 |
|
Ti |
0.7 |
The following procedure was applied in this experiment: a salt which is forming residue of the coprecipitant was added to 100-ml reference KDP solution, followed by its diffusion over the whole solution volume by stirring on the magnetic stirrer. The solution was then separated from the coprecipitant phase in the vacuum environment, using Buchner funnel. After that Al and Ti impurity concentrations were measured in the resulted solution using ICP-AES method, while Fe and Cr concentrations were measured using photocolorimetry analysis.
Determination of an optimal amount of the salt which is forming residue of the coprecipitant was based on the determination of its minimum amount sufficient for achieving minimum values of the relevant metals’ impurities concentrations in the solution, and not causing background concentration of additional contaminations, which are present in initial reagent, to occur in the KDP.
Impurity absorption efficiency is known to depend on environment acidity, so the selection of an optimal coprecipitant amount was followed by determining the best value of pH of the coprecipitation. Potassium hydroxide or phosphoric acid was added to the KDP solution to shift the pH. The coprecipitation degree was calculated using the Formula 1:
С = ((Meini-Mecop)/Meini) ∙ 100 % (1)
where С: coprecipitation degree, %
Meini: initial metal concentration in the reference KDP solution,
Mecop: metal concentration in the KDP solution after coprecipitation.
Results and Discussion
Coprecipitation with Metal Phosphates
A coprecipitant was added as a relevant metal’s salt solution with the concentration of 0.1 g/ml, expressed as cation. Adding this salt to the KDP solution caused the formation of white amorphous residue of corresponding metal’s phosphate. Following the reaction between coprecipitant phase and the solution, it was soaked for 24 hours to provide for complete precipitation and aggregating residue particles to ensure better filtration.
The amount of the coprecipitant added ranged from 0.1 to 2 % by weight. pH adjustment range was 4.3 to 6.0.
As Figure 1А shows, adding 1wt.% aluminum nitrate leads to quite complete impurity coprecipitation. Coprecipitation values close to maximum ones are achieved by adding 0.5 wt.% of the coprecipitant. Adding larger amount of aluminum is inappropriate, since it leads to contaminating the KDP solution with the aluminum nitrate impurities.
Calcium phosphate is an effective coprecipitant for iron ions, yet chrome and aluminum coprecipitation degree only slightly exceeded 65% (Fig. 1B). Adding the calcium nitrate in large amounts (>2 % by weight) also leads to reduced purification efficiency, which is likely to be due to coprecipitant contamination with initial reagent impurities.
Using zirconium phosphate coprecipitant achieved high iron and titanium coprecipitation degree, while those of aluminum and chrome were about ~ 60 % (Fig. 1C). These values of coprecipitation degree are achieved as early as when adding 1 % by weight of the coprecipitant.
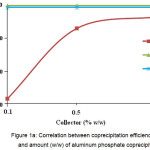 |
Figure 1a: Correlation between coprecipitation efficiency (%) and amount (w/w) of aluminum phosphate coprecipitant Click here to View figure |
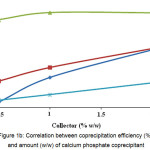 |
Figure 1b: Correlation between coprecipitation efficiency (%) and amount (w/w) of calcium phosphate coprecipitant Click here to View figure |
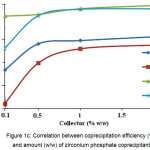 |
Figure 1c: Correlation between coprecipitation efficiency (%) and amount (w/w) of zirconium phosphate coprecipitant Click here to View figure |
The optimal pH value for applying aluminum and calcium phosphates is 5.0 (Fig. 2А and 2В). Micro-component coprecipitation with zirconium phosphate is appropriate at pH 4.3 (Table 2). An increase in pH has an adverse effect on the impurity coprecipitation, which is due to formation of more soluble form of zirconium phosphate and, as result, transition of initial reagent’s impurities to the KDP solution.
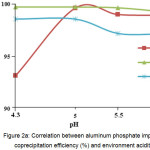 |
Figure 2a: Correlation between aluminum phosphate impurity coprecipitation efficiency (%) and environment acidity Click here to View figure |
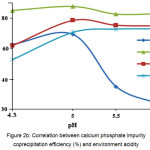 |
Figure 2b: Correlation between calcium phosphate impurity coprecipitation efficiency (%) and environment acidity Click here to View figure |
Table 2: Studied metals’ impurity content in the KDP solution after zirconium phosphate treatment at different pH values
|
Element |
Impurities content in the KDP solution, ppmw |
|||||
|
Reference solution |
pH |
|||||
|
2 |
4.3 |
5 |
5.5 |
6 |
||
|
Al |
5.0 |
6.5 |
2.4 |
17.2 |
57.0 |
77.9 |
|
Cr |
5.0 |
2.2 |
1.7 |
6.4 |
13.3 |
15.0 |
|
Fe |
5.0 |
1.5 |
0.2 |
0.8 |
8.2 |
9.5 |
|
Ti |
0.7 |
0.24 |
0.05 |
0.5 |
2.5 |
2.5 |
Coprecipitation with Hydrated Metal Oxides
Hydrated metal oxides were produced through reaction between ammonia solution and the relevant metal’s salt solution. As-precipitated hydroxide was separated from the mother solution in the vacuum environment, using Buchner funnel, followed by flushing with deionized water and adding to the KDP solution with the hydroxide amount of 0.5 to 5 % by weight (expressed as hydroxide weight). Required phase reaction time was about 1 hour, so coprecipitant phase could completely spread over the whole amount of KDP solution.
Maximum coprecipitation value, which is close to 100 % (of chrome, iron and titanium) is obtained when adding 5 wt.% of hydrated aluminum oxide (Fig. 3A).
When adding 5 wt.% of zirconium hydroxide, iron and titanium impurity coprecipitation degrees are about 80 %, while those of aluminum and chrome are about 40 % (Fig. 3В).
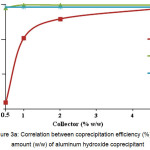 |
Figure 3a: Correlation between coprecipitation efficiency (%) and amount (w/w) of aluminum hydroxide coprecipitant Click here to View figure |
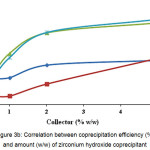 |
Figure 3b: Correlation between coprecipitation efficiency (%) and amount (w/w) of zirconium hydroxide coprecipitant Click here to View figure |
As Fig. 4 shows, the best aluminum hydroxide impurity coprecipitation takes place at pH value of 5.
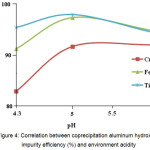 |
Figure 4: Correlation between coprecipitation aluminum hydroxide impurity efficiency (%) and environment acidity Click here to View figure |
The data on environment acidity impact on coprecipitation efficiency, which were obtained when adding 5 % by weight of the coprecipitant, are shown in Table 3. Increase in pH value leads to reduced efficiency of the studied metals’ impurity coprecipitation and contamination of the KDP solution with potassium hydroxide impurities, so an optimal value of environment pH is 4.3.
Table 3: Studied metals’ impurities content in the KDP solution after zirconium hydroxide treatment at different pH values
|
Element |
Impurities content in the KDP solution, ppmw |
|||||
|
Reference solution |
pH |
|||||
|
2 |
4.3 |
5 |
5.5 |
6 |
||
|
Al |
5 |
6.5 |
3.9 |
11.4 |
15.1 |
38.2 |
|
Cr |
5 |
2.8 |
3.0 |
5.7 |
6.4 |
8.1 |
|
Fe |
5 |
1.3 |
0.9 |
3.8 |
3.4 |
5.0 |
|
Ti |
0.7 |
0.2 |
0.1 |
0.5 |
0.6 |
0.7 |
The coprecipitant of layer-structure (vernadite, birnessite) hydrate manganese dioxide saturated with K+ cations was synthesized using technique mentioned in the relevant paper 10.
Performance of hydrate manganese dioxide (Mn-phase) is based on ion exchange sorption, so the reaction between reference solution and the coprecipitant was carried out in static environment, i.e. tightly sealed test tubes made of chemically inert material, while the ratio between coprecipitant weight and KDP solution volume was 1:100.
Contrary to the relevant technique10, the process was intensified by mixing Mn-phase and KDP solution in the magnet stirrer. The equilibrium is reached after 2-hour reaction, while coprecipitation degree of controlled impurities exceeds 90 % (Fig. 5).
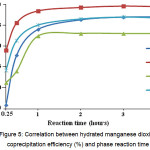 |
Figure 5: Correlation between hydrated manganese dioxide coprecipitation efficiency (%) and phase reaction time Click here to View figure |
Macro-Component Coprecipitant – KDP
Impurity coprecipitation with macro-component was studied using reference reagent with Al, Cr and Fe impurities concentrations of 5 ppmw and that of Ti of 0.7 ppmw.
The following procedure was applied in this experiment: such an oversaturation was created in the KDP solution that 10-50 % of initial reagent amount would precipitate during polythermal crystallization (Fig. 6). The crystals were separated from the mother solution in the vacuum environment, using Buchner funnel, and flushed with deionized water.
The efficiency of macro-component coprecipitant performance was assessed by purification degree (Formula 2) as follows:
K = Меini/Меmat (2)
where К: purification degree,
Meini: metal concentration in the initial KDP solution,
Memat: metal concentration in the KDP solution after coprecipitant precipitation.
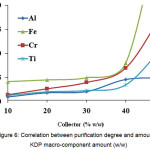 |
Figure 6: Correlation between purification degree and amount of KDP macro-component amount Click here to View figure |
An optimal coprecipitant amount is 30 to 40 % by weight, since using large amounts of the coprecipitant reduces potential economic effect during technology scaling.
Organic precipitators
The sodium diethyldithiocarbamate 0.01 to 0.5 % by weight of were added to the KDP.
This compound is an effective precipitator of Fe, Al and Ti ions (Fig. 7), while a certain decrease in chrome concentration in the solution is likely to be due to its sorption capture with developed surface of the residue being formed.
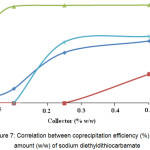 |
Figure 7: Correlation between coprecipitation efficiency (%) and amount (w/w) of sodium diethyldithiocarbamate Click here to View figure |
Cupferron was added as as-prepared 5% solution with its amount of 0.25 % by weight.
The most complete cupferron-triggered precipitation is registered against Fe and Ti impurities, as Table 6 shows. However, this precipitator is not suitable for precipitating Cr and Al ions, which is backed by reference data12. The shift in environment acidity values towards base or acid region hampers impurity coprecipitation, while potassium hydroxide and phosphoric acid solutions cause additional contaminations in the KDP solution (Table 4).
Table 4: Studied metals’ impurities content in the KDP solution after cupferron coprecipitant treatment at different pH values
|
Element |
Impurities content in the KDP solution, ppmw |
|||
|
Reference solution |
pH |
|||
|
2 |
4.3 |
6 |
||
|
Al |
5 | 7.20 | 6.5 | 5.7 |
|
Cr |
5 | 5.9 | 5.4 | 5.0 |
|
Fe |
5 | 2.25 | 0.02 | 6.3 |
|
Ti |
0.7 | 0.10 | 0.03 | 1.1 |
Summarized experiment data, including absolute residual post-coprecipitation metal concentration values are shown in Table 5.
Table 5: Summary coprecipitant’s performance table
|
|
Impurities content in the KDP solution, ppmw |
|||||||||
|
|
Coprecipitants |
|||||||||
|
Element |
Reference solution |
Phosphates |
Hydrated oxides |
KDP macro-component |
Organic precipitators |
|||||
|
Aluminum phosphate |
Calcium phosphate |
Zirconium phosphate |
Aluminum hydroxide |
Zirconium hydroxide |
Manganese hydroxide |
Sodium diethyldithiocarbamate |
Cupferron |
|||
|
Al |
5.0 |
– |
1.3 |
2.4 |
– |
3.9 |
0.25 |
1.6 |
3.0 |
5.0 |
|
Cr |
5.0 |
0.02 |
0.6 |
1.7 |
0.7 |
3.0 |
0.04 |
1.0 |
5.0 |
5.0 |
|
Fe |
5.0 |
0.02 |
0.1 |
0.2 |
0.15 |
0.9 |
0.25 |
1.1 |
0.02 |
0.02 |
|
Ti |
0.7 |
0.01 |
0.1 |
0.05 |
0.02 |
0.1 |
0.02 |
0.4 |
0.3 |
0.03 |
The most complete coprecipitation of iron, chrome and titanium impurities is reported when using 0.5 % by weight (pH: 5) of aluminum phosphate as a coprecipitant. At the same time the concentration of the impurities mentioned is reduced by more than 2 points (Table 5). According to the reference data, it can be assumed that during metal phosphate the coprecipitation substitution solid solutions are formed, when impurity ions move to crystal phase’s lattice points, due to the crystal-chemical affinity between coprecipitant and macro-component phases.
Effective removal of iron and titanium is reported during 1 % by weight (pH: 4.3) zirconium phosphate precipitation, while their post-purification concentration values are 0.2 ppmw and 0.05 ppmw respectively (Table 5).
Almost complete iron and titanium coprecipitation is also reported when using aluminum hydroxide coprecipitant added by 1 % by weight (pH: 5) (final impurity concentration values are 0.15 ppmw and 0.02 ppmw respectively) and also during precipitation with 0.25 % by weight of cupferron (post-purification impurity concentration values are: 0.02 ppmw and 0.03 ppmw respectively) (Table 5).
Impurities coprecipitation with aluminum hydroxide is due to absorption and isomorphic capture. Also, given the fact that the micro-component is found in the solution in the form of charged phosphate complexes of Fe(HPO4)+ 14 compound, iron’s capture by metal hydroxide residue is likely to follow acid-base reaction between iron and coprecipitant complexes. Cupferron precipitation is accompanied by formation of slightly-soluble salts, in which a metal substitutes ammonia ion and coordinately binds to the nitrozo group.
It is hydrated manganese dioxide that is a universal coprecipitant of all controlled impurities. The ratio between coprecipitant weight and solution volume used was 1:100. Maximum coprecipitation (of about 90 %) is achieved through 2-hour reaction between coprecipitant phase and KDP solution.
Metal cations are absorbed by the whole volume of poorly-ordered structures of hydrated manganese dioxide, which is accompanied by their equivalent inter-substitution of potassium cations from the relevant phases.
Macro-component coprecipitant is an effective coprecipitator of the impurities in question, which does not cause additional contaminations in the KDP. An optimal coprecipitant amount is 30 to 40 %.
Conclusions
The experiments aiming at selecting the most effective coprecipitants were carried out using reference KDP solution. It was shown that using aluminum phosphate as a coprecipitant of Fe, Cr and Ti impurities allows to reduce their concentrations by more than 100 times. To reduce Fe and Ti concentration values from 5 ppmw and 0.7 ppmw respectively it is efficient to use zirconium phosphate (to 0.2 ppmw and 0.05 ppmw respectively), aluminum hydroxide (to 0.15 ppmw and 0.02 ppmw respectively) and cupferron (to 0.02 ppmw and 0.03 ppmw respectively) as coprecipitants. Precipitation with sodium diethyldithiocarbamate and calcium phosphate reduced iron content to 0.1 ppmw and 0.02 ppmw respectively.
It was found that hydrated manganese dioxide is a universal coprecipitant of Al, Fe, Cr and Ti impurities, which ensures reducing Al, Fe, Cr and Ti concentration values to 0.25 ppmw, 0.25 ppmw, 0.04 ppmw and 0.02 ppmw respectively. KDP macro-component is an effective coprecipitant for the impurities in question, which also does not cause additional contaminations in the KDP solution (final concentration values after one-stage purification: Al, Cr and Fe: about 1 ppmw, Ti: about 0.4 ppmw).
Coprecipitants amounts required for achieving minimum values of impurities content were selected (Al, Cr and Fe ≤ 0.5 ppmw, Ti ≤ 0.07 ppmw). An optimal amount of aluminum phosphate and hydrated manganese dioxide is 1 % by weight, while that of aluminum hydroxide and zirconium phosphate is 0.5 % by weight, and that of organic precipitators is 0.25 % by weight.
It is appropriate to precipitate 40 % of macro-component coprecipitant.
Environment pH values that ensure the best impurity coprecipitation were determined. In case of coprecipitation with coprecipitants based on aluminum compounds and calcium phosphate, an optional pH value is 5. The most efficient purification by zirconium phosphate and cupferron coprecipitants is achieved at pH value of 4.3.
Acknolegements
Current work is supported by grant of Ministry of Education and Science of Russia 14.625.21.0039, project identifier RFMEFI62516X0039.
References
- Andreev, N.; Babin, A.; Bredikhin, V.; Ershov V. Fotonika. 2007, 5, 34-37.
- Voronov, A. P.; Babenko, G. N.; Puzikov, V. M.; Roshal, A. D.; Salo, V.I. Crystallography Reports. 2008, 53, 708-712.
CrossRef - Voronov, A. P.; Salo, V. I.; Puzikov, V. M.; Tkachenko, V. F.; Vydai, Y. T. 2006, 51, 696-701.
- Eremina, T. A.; Kuznetsov, V. A.; Eremin, N. N. Crystallography Reports. 2002, 47, 76-85.
CrossRef - Patnaik, P. McGraw-Hill. 2003, 1086.
- Kurmanguzhina, L. K.; Marov, I. N.; Evtikova, G. A.; Fakeev, A. A.; Knyazeva, A. N. Journal of Inorganic Chemistry. 1982, 27, 1490-1493.
- Kurmanguzhina, L. K.; Marov, I. N.; Evtikova, G. A.; Fakeev, A. A. Journal of Inorganic Chemistry. 1992, 37, 1011-1014.
- Kurmanguzhina, L. K.; Marov, I. N.; Kalinichenko, N. B.; Fakeev, A. A. Journal of Inorganic Chemistry. 1993, 38, 968-970.
- Kurmanguzhina, L. K.; Marov, I. N.; Evtikova, G. A.; Fakeev, A. A. Journal of Inorganic Chemistry. 1983, 28, P. 2538.
- Novikov, G. V.; Kulikova, L. N.; Bogdanova, O. Yu.; Sychikova, G. I.; Dara, O. M. Journal of Inorganic Chemistry. 2005, 50, 1972-1980.
- Efremova, E. P.; Sukhanovskaya, A. I. and Kuznetsov, V. A. Inorganic materials. 2004, 40, 730-734.
CrossRef - Kuzmin, N. M., Zolotov, Yu. A. Nauka. 1988, 64-65.
- Dosovitsky, A. E.; Komendo, I. Yu.; Mikhlin, A. L.; Retivov, V. M.; Bulatitsky, K. K.; Rodchenkov, V. I. Zavodskaya Laboratoriya. Diagnostika materialov. 2017, 83, 13-17.
- Efremova, E. P.; Kuznetsov, V. A.; Klimova, A. Yu. Crystallography. 1993, 38.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









