Phytochemical Composition, Antioxidant and Antimicrobial Activities of Ammodaucus Leucotrichus Fruit from Algerian Sahara
Asjel Sebaa1 , Abderazek Marouf2, Nadia Kambouche1 and Aicha Derdour1
, Abderazek Marouf2, Nadia Kambouche1 and Aicha Derdour1
1Organic Synthesis laboratory, Department of Chemistry, Faculty of Sciences, University of Oran 1 Ahmed Benbella, P.B. 1524, El M'Naouer, 31000 Oran, Algeria.
2Institute of Science and Technology, Department of Natural Sciences and Life, Ctr Univ Naama, P.B. 66, 45000 Naama, Algeria.
Corresponding Author: E-Mail: lamia.sebaa@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/340158
Ammodaucus leucotrichus Cosson & Durieu common appellation is “Nessoufa, Moudrayga”. It is an endemic plant from the family Apiaceae (Umbelliferae) frequently used by the Arab world for traditional medicine and as a food condiment. A. leucotrichus fruits have been phytochemically screened to evidence qualitative composition of secondary metabolites. Analyses assess the presence of flavonoids, tannins, saponins, coumarins, phenolic acids, alkaloids and cardiac glycosides. Antioxidant activity of different extracts is evaluated by using two methods: Ferric Reducing Antioxydant Power (FRAP) and DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical capture. Methanolic extract has a good ferric reducing capacity, superior to the capacity recorded through BHT. The capacity of trapping of free radical DPPH is interesting with an IC50 =2,256 µg/ml, which is similar to BHA with an IC50=2.167µg/ml. The extracts and fractions are also evaluated for their antimicrobial activity against eight bacterial strains and three human pathogenic fungi, exhibiting an interesting antimicrobial profile.
KEYWORDS:Ammodaucus Leucotrichus; Fruit; Polyphenols; Antioxidant Activity; Antimicrobial Activity
Download this article as:| Copy the following to cite this article: Sebaa A, Marouf A, Kambouche N, Derdour A. Phytochemical Composition, Antioxidant and Antimicrobial Activities of Ammodaucus Leucotrichus Fruit from Algerian Sahara. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Sebaa A, Marouf A, Kambouche N, Derdour A. Phytochemical Composition, Antioxidant and Antimicrobial Activities of Ammodaucus Leucotrichus Fruit from Algerian Sahara. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42558 |
Introduction
The world has an endless quantity of plants used for multiple purposes. For certain plants, medicinal properties are more important than food, condiment or ornament use. Several studies assessed that therapeutic effects of certain plants are related to the presence of chemical substances such as essential oil, saponins, flavonoids and alkaloids. These metabolites are interesting because of their biological and pharmacological proprieties as well as their industrial production. For instance, The Algerian flora with its 3000 species belonging to several botanical families Including 15% endemic, remains very little explored on the phytochemical plan as on the pharmacological plan. The valorization of the Medicinal Plants of the national flora will be a great contribution to the pharmaceutical industry of Algeria and will have an economic impact certain1. Ammodaucus leucotrichus Coss. & Dur. is from Leucotrichus subsp. and Apiaceae family is referred to as ‘Kamune es sufi or akâman’ in most of the Arabic-African countries while in Algeria, it is commonly known as “Moudrayga” and known as “Cumin chevelu” in French 2-3. It is a small glabrous annual plant which Stems are locally raised, streaked and small branches shaped. Leaves are fleshy, finely divided forming flat narrow ridges. Umbels have 2 to 4 rays and involucres have very divided bracts. White flowers are positioned according to a composed umbel. Mericarps are 6-9 x 4-5mm long with secondary ribs covered with 8 to 10mm long silky hair. Hair is very dense and fuzzy, with a yellow reddish base and a white end4. The plant has a strong smell of anise. A. leucotrichus is an aromatic plant endemic of North Africa (Algeria, Morocco, Tunisia, Libya, Egypt, Mauritania and upper Niger valley) 3-5. Its best implantation is in desert regions, often down a hill or a dune.In the Algerian traditional medicine, the fruits from A. leucotrichus are used either by the local population to the treatment of common cold, fever, vomiting, stomach ache and allergies particularly with children6. Previous phytochemical investigations on this plant are limited. Bernard et al. reported the isolation and characterization of four compounds: ammolactone-A (1), ammolactone-B (2), dihydroxy acid (3) and 3-hydroxyperil aldehyde (4)2. The purpose was to conduct a phytochemical and biological study concerning this plant well known in Southern Algeria for its medicinal virtues. This is why we planned to phytochemically screen different extracts and evaluate the antioxydant potential, antimicrobial.
Materials and Methods
Plant Material
The plant material consists of fruits from Ammodocus leucotrichus Coss. et Dur. harvested in the region of Bechar, (south of Algeria). The authentication was performed by Professor A. Marouf (Institute of Science and Technology, Department of Natural Sciences and Life, Ctr Univ Naama, Algeria). Dried fruits were pulverized through a crusher to get a fine powder. Powder was used to prepare different extracts.
Preparation of Extracts
Powdered dried fruits (each 5 g) were extracted with 50 ml each of dimethyl ether, methanol 80%, ethanol 70% and Aqueous individually, at ambient temperature, with agitation for 24–48 h. Three extracts were obtained (EM1, EM2, EED and EE70%). The extracts were concentrated under pressure and the residues containing water were dried by lyophilisation.
Twenty five grams of the extract ethanolic 70% were included in 200 ml of distilled wateradded with lead acetate [(CH3COO)4 Pb] to remove chlorophyll and other low molecular weight compounds by precipitation. After filtration, the solution became red-brown. The filtrate was subjected to sequential extraction with dichloromethane, ethyl acetate and n-butanol. Thus, three obtained organics phases (DMC, AcOEt and BOH) were dried by sulfate anhydrous sodium, then filtered, concentrated and dried under reduced pressure.
An essential oil was obtained through an 8 h hydro-distillation. The distilled plant material consists of A. leucotrichus dried fruits. The oil was dehydrated with anhydrous sodium sulfate and stored at 4°C in the dark.
Chemical and Reagents
Ether dimethyl, CHCl3, CH2Cl2, 70% Ethanol, 80% methanol, n-butanol, ethyl acetate, (DMSO) dimethyl sulfoxide, Distilled water, (FRAP) Ferric Reducing Antioxydant Power, DPPH (2, 2-diphenyl-1-picrylhydrazyl), BHT, BHA, Quercetin, Ascorbic acid, Acetic acid, formic acid, lead acetate, NH4OH Potassium hydroxide, phosphate buffer, Potassium ferricyanide, trichloroacetic, SbCl3, Fehling solution, Folin-Ciocalteu solution, Dragendorff, Komarowski, Strasny, Neu, anisaldehyde and Zimmermann reagents, TLC plates 60F254 silica, Potassium hydroxide, Ferric chloride, Muller Hinton medium (MHI), Sabouraud medium and Sulfate anhydrous sodium were obtained from Sigma Aldrich Chemicals and other chemicals and reagents used were of analytical grade.
Preliminary Phytochemical Analysis
Screening was processed with chemical tests and/or chromatographic tests through 60F254 silica TLC assays after disclosing compounds with specific reagents and after examining results through visible light and/or UV7-8. Migration systems used were:AcOEt-MeOH-H2O (100: 13.5: 10) (Anthracenes derivatives, cardiac glycosides); AcOEt-AcOH-HCOOH-H2O (100:11:11:26) (coumarin, phenol acid, flavonoids, sesquiterpenelactones); CHCl3-MeOH-H2O (65:35:10) (saponins); CH2Cl2-MeOH-NH4OH(95:5:0,5) (alkaloids); AcOEt-MeOH-H2O (100 :17 :13) (quinones). For TLC plates disclosing, reagents used were: Folin-Ciocalteu (phenol acid), Reducing compounds (Fehling), Dragendorff (alkaloids), Komarowski (saponins), Neu (flavonoids), Strasny (tannins), anisaldehyde (sterols, triterpenes), Zimmermann (lactone sesquiterpenes), KOH (anthraquinones, coumarins, quinones), SbCl3 (cardiac glycosides).
Antioxidant activity
Reducing power method
Reducing power of methanol extract of A. leucotrichus fruits was measured according to Yen & Chen method 9. Briefly, 1.0 ml of different concentration sample (0.25, 0.5,0.75 and 1.0 mg/ml) was mixed with 2.5 ml of a 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of a 1% (w/v) solution of potassium ferricyanide. The mixture was incubated in a water bath at 50°C for 20 min. afterwards, 2.5 ml of a 10% (w/v) trichloroacetic acid solution was added and the mixture was then centrifuged at 10000 rpm for 10 min. A 2.5 ml aliquot of the upper layer was combined with 2.5 ml of distilled water and 0.5 ml of a 0.1% (w/v) solution of ferric chloride, and absorbance was measured at 700 nm. BHT (0.25, 0.5, 0.75 and 1 mg/ml concentrations) was used as a positive control.
DPPH Method
DPPH method was used to assess anti-free radical activity of extracts different10-11. A 6 10-5M solution of DPPH in methanol was prepared and 1950µl of this solution was added to 50µl of various concentrations (10, 100, 200, 400 and 500 µg/ml) of samples dissolved in methanol to be tested. BHA, ascorbic acid and quercetin, were used as standard. After 1h 30 min, of incubation in a dark room, absorbances were read at 517 nm. The scavenging inhibitory effect of DPPH was calculated according to the following formula: DPPH radical scavenging activity (%) = [(Absorbance of control–Absorbance of sample)/Absorbance of control] × 100 The concentration of the test extracts providing 50% inhibition (IC50, expressed in μg/ml) were calculated from the graph plotted with inhibition percentage against the extracts concentration. All determinations were performed in triplicate.
Antimicrobial activity
The bacterial cultures used in this study are of clinical origin (isolated and identified by the Service from Bacteriology, Hospital EHU of Oran, Algeria); they are eight bacteria and three fungi strains: Escherichia coli ATCC 25922, pseudomonas aeruginosa ATCC 27853, Klebsilla pneumonia ATCC 13883, Salmonella typhimurium ATCC 13311, Proteus vulgaris ATCC 13315, Bacillus cereus ATCC 14579, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29912, Candida albicans ATCC 10231, Aspergillus niger ATCC 16404 and Trichophyton rubrum ATCC 28188.
The anti-microbial activity test, carried out on the various fractions and the extracts of the fruits of A. leucotrichus Coss. et Dur. , was determined using the disk diffusion method12-13. Bacteria species were cultivated for 24 h in Muller Hinton medium (MHI) at 37°C and for 48 hrs at 25°C in Sabouraud medium for fungi. The suspension of the tested microorganisms (approximately 106 CFU/ml) was spread on the solid media plates (20µl). A sterile 6-mm-diameter Filter disk (WATTMAN paper N° 3) was impregnated with 10 μL of serial dilutions in dimethyl sulfoxide (DMSO) of the various fractions and the extracts (EM1, EM2, EE70%, EED, DMC, and BOH), and was placed onto the solid media plates. The diameter of inhibition was measured after 24 or 48 h of incubation at 25°C or 37°C. Standard antibiotic CN (Céfalexine 10 μg) was used in order to control the sensitivity of the tested bacteria. The antimicrobial activity was assessed by measuring the zone of growth inhibition surrounding the disks.
Results and Discussion
Knowledge of phytochemical composition of plants is important in discovery of therapeutic agents for treatment and prevention of various diseases. The present work focused essentially on the phytochemical, antioxidant and antibacterial studies from A. leucotricus Coss. et Dur. The specie has been screened for 12 chemical groups by reactions colored and results are represented in table 1. It is worth noting that the presence of Quinones, Anthracenes compounds, Sterols and triterpenes, Reducing compounds, Phenol acids and Cardiotonic glucoses has not been previously reported in the literature. The presence of such metabolites indicates importance of plant extracts, for examples, flavonoids are considered important due to their antioxidant and antimicrobial activities14.
Table 1: Phytochemical screening by color reactions of A. leucotrichus fruits.
| Compound groups | Tests | Results |
| -Tannin | Stiasny | + |
| -Quinones : Free quinonesCombined quinones | HCl,1N,CHCl3-NH4OHH2SO4 reflux CHCl3 |
– – |
| -Anthracenes compounds: Free anthracenesCombined anthracenes | CHCl3/NH4OH ExtractCHCl3/FeCl3 Extract |
+ ++ |
| -Sterols and triterpenes | Libermann burchard | +++ |
| -Sesquiterpene lactones | Aqueous extract /CHCl3 | – |
| -Reducing compounds | Fehling | + |
| -Alkaloids | Extraction in acid mediumExtraction in alkaline medium | -+ |
| -Phenol acids | Folin-Ciocalteu |
Cinnamic acid Ferulic acid |
| -Cardiotonic glucoses-Saponins-Flavonoids-Coumarins | AcOPb extractionCH2Cl2 extractMeOH extractMeOH extract | ++++++++++ |
Key: (-) absent; (+): low quantity; (++): average quantity; (+++): high quantity.
The result of antioxidant activity by the ferric reducing was detected in all the extracts: weak in the aqueous extract, oils essential and strong in the extract methanolic of A. leucotrichus fruits. The reducing ability of a compound generally depends on the presence of reductants15 which have been exhibiting antioxidative potential by breaking the free radical chain and donating a hydrogen atom16. The capacity of the methanolic extract to reduce Fe+3 ions into Fe+2 ions was determined (Figure 1), this antioxidant activity of methanolic extract weak compared to reference compound (BHT) in 0.25 mg/ml to 0.75 mg/ml concentrations, but starting from 1mg/ml concentration, antioxidant activity is much better (140%).
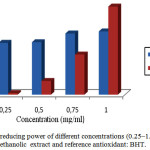 |
Figure 1: Total reducing power of different concentrations (0.25–1.0 mg/ml) of methanolic extract and reference antioxidant: BHT. Click here to View figure |
All the extracts (methanol, water and essential oil) showed a remarkable quantity of antioxidant activity, although the values were quite less than the references antioxidants (BHA, Ascorbic acid, Quercetin). Free radical scavenging activity of all the extracts and references antioxidants increased with the increase in concentration. The maximum percentage inhibition of DPPH free radical at 517 nm is exhibited by ascorbic acid about 93.56 to 99.00%; followed by methanol extract, Quercetin, BHA, essential oil and aqueous extract of A. leucotrichus as shown in table 2.
It is implied that plant extracts contain compounds such as phenols, flavonoids, etc., which can donate hydrogen to a free radical in order to remove odd electron indicating its usefulness in various radical related pathological condition; pattern of inhibition being similar at variable concentration due to the fact that it possesses high radical scavenging activity, that is, this concentration is sufficiently high to scavenge free radicals. Methanol extract of A. leucotrichus has shown the highest activity followed by essential oil and water extract. The antioxidant activity shown by methanol extract was much higher than that of oil essential which may be due to the presence of polar compounds like phenols, flavonoids, etc., that are soluble in methanol17.
Table 2: DPPH radical-scavenging activity of A. leucotrichus fruits.
| Samples | Pourcentage d’inhibition du radical DPPH (%) a | |||||
| 10 (µg/ml) | 100 (µg/ml) | 200 (µg/ml) | 400 (µg/ml) |
500 (µg/ml) |
||
| MeOH extract | 4.35±1.22 | 50.57±2.01 | 89.29±1.11 | 93.31±4.87 |
98.16±0.15 |
|
| E.Oil | 26.45 | 27.52 | 36.17 | 65.51 |
78.91 |
|
| A.P extract | 11.22±0.11 | 18.31±0.55 | 26.89±2.60 | 35.47±0.18 |
65.18±1.13 |
|
| BHA | 31.18±1.02 | 38.11±4.80 | 69.30±1.17 | 92.07±0.07 |
94.88±0.01 |
|
| Ascorbic acid | 25.57±0.29 | 40.92±0.99 | 93.56±6.90 | 99.00±0.36 |
99.00±0.58 |
|
| Quercétine | 35.47±1.28 | 71.61±0.62 | 97.85±0.22 | 97.85±0.54 |
97.85±0.95 |
|
aMean ± SD (n=3)
As shown in table 2, antioxidant activity dosing by using DPPH whitening assesses that methanol extract has the best performance, particularly in 500 concentrations where the activity is equivalent to positive indicators: BHA, ascorbic acid and quercetin. IC50 value settled for every sample is stated as being the substrate concentration losing 50% of DPPH activity (table 3). Lower IC50 value indicates higher antioxidant activity.
Table 3: Estimated values of inhibiting concentrations IC50 of fruits from A. leucotricus
| Samples | Equation de la courbede tendance adoptée | ConcentrationIC50 (μg/ml) | Coefficient deDétermination R2 |
| MeOH extract | y=23.03x- 01.96 |
2.256 |
0.835 |
| E.Oil | y=14.29x+04.03 |
3.216 |
0.896 |
| A.P extract | y=12.50x- 06.11 |
4.486 |
0.890 |
| BHA | y=18.13x+10.70 |
1.495 |
0.936 |
| Ascorbic acid | y=20.49x+10.13 |
1.945 |
0.832 |
| Quercetin | y=15.10x+34.82 |
2.167 |
0.757 |
All extracts have a free radical reduction capacity. Required concentrations to neutralize and stabilize 50% of DPPH concentration goes from 2 to 4 µg/ml. Comparing results from reference compounds (BHA, Quercetin and Ascorbic acid), we assess that methanol extract activity is quite similar to BHA activity and that extracts antioxidant capacity is decreasingly arranged as follows: Methanol extract > E.oil > Aqueous extract of aerial part.
In this study, we evaluated the antimicrobial activity of the various fractions and extracts of fruits from A. leucotricus Coss. et Dur. on the in vitro growth of eight bacterial strains and three fungi strains. As shown in Table 4, the inhibition zones of disc for strains were in the ranges 7.0 – 18.0 mm. Generally, the extracts of the plant were moderately active against Gram positive and negative bacteria. This was followed by DMC, BOH, EEM1, EE70%, EEM2 and EED extracts respectively. The growth of E. coli and S. aureus were inhibited by different fractions and extracts. The results against the fungic stocks are interesting (table 4), because all the extracts and fractions from A. leucotrichus announced a antifungal activity and the greatest effect was obtained by fraction BOH on T. rubrum and extract EM2 on C. albicans whose zones of inhibition were of 20 and 15 mm respectively, contrary to A. niger only the extract EM2 and fraction BOH gave an inhibition. When comparing the antibacterial activity of the tested extracts to that of reference antibiotic, Céfalexine, their inhibitory potency was not found to be significant. The maximum antimicrobial activity against B. cereus and T. rubrum of fractions DMC and BOH could be explained by the presence of various components, in particular, the flavonoïdes, tannins, the phenolic acids, and terpenes whose antimicrobial properties have been demonstrated by several researchers18-19.
Table 4: Antimicrobial activity of different fractions and extracts of A. leucotrichus fruits.
| Microbial strain a | |||||||||||
| Samples | E. coli. | S. typhimurium | P. vulgaris | K. pneumonia | P. aeruginosa | B. cereus | S. aureus | E. faecalis | C. albicans | A. niger | T. rubrum |
| 1-EM1 | nd | 8 | 8 | 7 | 6 | 6 | 8 | 11 | 12 | 6 | 12 |
| 2-EM2 | 6 | 6 | 7 | 9 | 9 | 6 | 6 | nd | 15 | 10 | 8 |
| 3-EED | 6 | 6 | 8 | 8 | 6 | 6 | 6 | 6 | 10 | 6 | 8 |
| 4-EE70% | 7 | 6 | 7 | 16 | 7 | 6 | 6 | nd | 8 | 6 | 12 |
| 5-BOH | 7 | 9 | 7 | 14 | 9 | 17 | 9 | 6 | 8 | 8 | 20 |
| 6-DMC | 8 | 8 | 7 | 16 | 11 | 18 | 6 | 6 | 10 | 6 | 18 |
| Céfalexine b | 18 | 16 | 28 | 36 | 16 | 32 | nd | 6 | – | – | – |
EM1: Methanolic extract (24h); EM2: Methanolic extract (48h); EED: Dimethyl ether extract; EE70%: Ethanolic extract 70%; DMC: Dichloromethanic fraction; BOH: Butanic Fraction. nd: not determined.
aInhibition zone, including diameter of the paper disc (6 mm).
bReference antibiotic.
Conclusion
Results are directly related to quantitative and/or qualitative diversity of compounds’ content in extracts. It is important to be aware that these results were obtained only in vitro. The interesting fact is that we can then directly study compounds’ or extracts’ antioxidant activity in vivo to correlate results recorded in both cases. Further studies on other different micro-organisms will help in the evaluation of the therapeutic potential of this plant. These results of antimicrobial assays justified and supported partly the popular usage of the all organs, especially fruits as traditional remedies for some infections.
Acknowledgments
The authors wish to thank the Ministry of Higher Education and Scientific Research of Algeria for financial support (Project Cnepru No. E1820130013).
References
- Ozenda, P. Flore and vegetation of the Sahara. 3th edition, editions of the national center of scientific research. (2004).
- Bernard, M.; Fouzia, D.; Didler Le, N.; Souad, FT and Jean-Pertte, R. Phytochem, 1997, 44, 907-910.
CrossRef - Maberly, P.L. The Plant Book. Cambridge University Press, Cambridge, UK. (1998).
- Quezel, P and Santa, S. Nouvelle flore de l’Algérie et des régions désertiques méridionales. Edition du centre national de la recherche scientifique. (1963).
- Hammiche, V.; Maiza, K . J. Ethnopharmacol. 2006, 105, 358-367.
CrossRef - Velasco-Negueruela, A.; Pérez-Alonso, M.J, Pérez de Paz, P.L.; Palá-Paúl, J and Sanz, J. J. Chromatogr. A. 2006, 1108, 273-275.
CrossRef - Fry, S.C. The Growing Plant Cell Wall: Chemical and Metabolic Analysis, Longman scientific and technical, UK. (1988).
- Wagner, H and Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. Springer-Verlag: Berlin. Heidelberg, New York. (1996).
CrossRef - Yen, G. C and Chen, H. Y. J. Agric. Food Chem. 1995, 43, 27-32.
CrossRef - Takao, T.; Kitatani, F.; Watanabe, N.; Yagi, A and Sakata, K. Biosci. Biotechnol. Biochem. 1994, 58, 1780-1783
CrossRef - Roberto Lo Scalzo. Food Chem. 2008,107, 40-43.
CrossRef - Sacchetti, G.; Maietti, S .; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M and Bruni, R. Food Chem. 2005, 91, 621-632.
CrossRef - Celiktas, O.Y.; Kocabas, E.E.H.; Bedir, E.; Sukan, F.V.; Ozek, T and Baser, K.H.C. Food Chem. 2007, 100, 553-559.
CrossRef - Baydar, H.; Sagdic, O.; Ozkan, G and Karadogan, T. Food Control. 2004, 15, 169-172.
CrossRef - Duh, P.D.; Tu, Y.Y and Yen, G. C. Technol. 1999, 32, 269-277.
- Gordon, M.H. The mechanism of the antioxidant action in vitro, In: Food antioxidants, (ed) B.J.F. Hudson, Elsevier, London. (1990).
- Bhusal, A.; Jamarkattel, N.; Shrestha, A.; Lamsal, N.K.; Shakya, S.; Rajbhandari, S. J. Clin. Diagn. Res. 2014, 8: HC05.
- Bisignano, G.; Tomaino, A.; Lo Cascio, R.; Crisafi, G.; Uccella, N and Saija, A. J. Pharm. Pharmacol. 1999, 51, 971- 4.
CrossRef - Markin, D.; Duek, L and Berdicevsky, I. Mycoses. 2003, 46, 132-136.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









