Optical Pattern of Liquid Crystalline Structure in Singular and Mixed Anionic-Cationic Surfactant System
Laili C.R , Doreen N. S and Hamdan S.
, Doreen N. S and Hamdan S.
School of Fundamental Science, Universiti Malaysia Terengganu, 21030, K.Terengganu, Terengganu, Malaysia.
Corresponding Author E-mail: laili@umt.edu.my
DOI : http://dx.doi.org/10.13005/ojc/340112
A preliminary study to examine the liquid crystalline region formed by surfactant, DTAB and AOT, individually and mixed, in the presence of various chain length alcohols was carried out. Several ternary phase equilibrium were constructed. Results showed that all the investigated systems apart from forming lyotropic liquid crystalline structure were also able to form self-assembled aggregates such as micelle and reversed micelles. From the optical pattern observation, it was observed that two types of liquid crystalline were formed namely hexagonal and lamellar.
KEYWORDS:Phase Diagram; Optical Pattern; Surfactants
Download this article as:| Copy the following to cite this article: Laili C. R, Doreen N. S, Hamdan S. Optical Pattern of Liquid Crystalline Structure in Singular and Mixed Anionic-Cationic Surfactant System. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Laili C. R, Doreen N. S, Hamdan S. Optical Pattern of Liquid Crystalline Structure in Singular and Mixed Anionic-Cationic Surfactant System. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41472 |
Introduction
When a surfactant adsorbs from aqueous phase at a hydrophobic surface, the molecule’s hydrophobic group causes the molecule to concentrate at the surface with the orientation where the hydrophobic group towards the surface and exposes the hydrophilic group to the aqueous phase. Since the hydrophobic group has lower surface tension than that of the aqueous phase, the addition of surfactant resulted in great reduction of surface tension1-5. At some point, the distribution of the surfactant molecules at the surface would reach equilibrium where the surface tension will become almost constant. At that point onwards, the continuous addition of surfactant will not have any effect on the surface tension as it will remain virtually unchanged. At normal circumstances, phase separation will occur just like in the case where water is saturated with polar substances. However, in the case of surfactant, the molecules will self-assembled to create a microphase in which the hydrophobic groups will be directed towards the interior of a cluster to minimize the contact with the surrounding while the hydrophilic groups are oriented towards the aqueous media. The cluster is normally known as the micelle, while the point at which micellization initiates is denoted as the critical micelle concentration (CMC)2. Further addition of surfactants, this micelle will then turn change to liquid crystals6 and other association structures. Liquid crystals structure is crucial as it resembles the structure of the topmost layer of the skin previously described7.
In view of this process, it is important to understand that self-assembled aggregation is a physico-chemical process. However in many industrial application singular surfactant can afford all the requirement needed in a product. Therefore, a mix surfactant systems becomes essential and have shown improvement in the product performance8-14.
Experimental
Construction of phase diagrams
The phase diagrams were determined on a clear/turbid criteria basis by mixing two of the components and titrating with the smallest amount of the third component. The samples were then thoroughly mixed to homogeneity with a vortex mixer, centrifuged and then allowed to reach equilibrium at a specific temperature in a water bath. The phases were then examined by visual inspection between cross polarisers. An estimated region of the phases can then be made by this method by noting the turbid and clear compositions.
Polarised Optical Microscopy
The liquid crystalline phases were studied and differentiated by polarizing optical microscopy since hexagonal and lamellar phases exhibited anisotropic properties and characteristic optical texture under crossed polarizer. The studies were performed by Olympus Provis AX70 equipped with polarizing filters and PM30 automatic photomicrography system that provides fully automatic exposure control for fluorescence photomicrography. Thin film of lyotropic phases were prepared on microscope slide and covered immediately with cover slide to prevent water evaporation. Sample slide was placed under the stage. Magnification of 200x were used and photomicrographs of various optical pattern were captured by the camera.
Result s and Discussion
The results obtained from the studies will be presented and discussed in two parts. The first part involved the ternary and pseudo phase equilibriums whereby the constructed phase diagrams in the study were presented. While explaining the phase behaviour of the surfactants, optical patterns of the liquid crystalline observed in the phase equilibrium that were captured by the polarized optical microscope were also presented.
Phase Diagrams
DTAB/ Alcohol /H 2 O Systems
In order to examine the effect of the chain length of co-surfactant on the urfactant’s phase behavior as well as to determine the suitable co-surfactant for aqueous DTAB, a series of ternary phase equilibrium consisting DTAB and water with butanol (C4-OH), hexanol (C6-OH), octanol (C8-OH), decanol (C10-OH), dodecanol (C12-OH) and hexadecanol (C16-OH) were constructed. The resulting series of phase diagram was as shown in Figure 1.
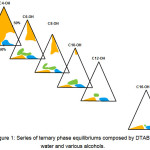 |
Figure 1: Series of ternary phase equilibriums composed by DTAB, water and various alcohols. Click here to View figure |
From the overview, it was found that, the DTAB/alcohol/H2O systems phase equilibrium were able to form two isotropic dispersions namely micelle and reversed micelle, where micelle was formed at the alcohol apex while the reversed micelle was formed at the water apex. In addition to that, the formation of liquid crystalline was detected in the all phase diagrams. The hexagonal liquid crystalline region was situated at the water-DTAB axis, closer to the DTAB apex while lamellar liquid crystalline region was located in between the water-DTAB axis and water-alcohol axis, closer to the water apex.
DTAB/AOT/H2O System
The ternary phase equilibrium of DTAB/AOT/Water system was constructed to examine the phase behavior of DTAB and AOT mixture in water and consecutively, to determine the ratio at which the mixtures of DTAB and AOT best performed. The resulting phase equilibrium of the DTAB/AOT/Water system was shown in Figure 2.
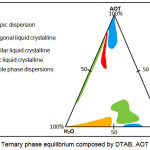 |
Figure 2: Ternary phase equilibrium composed by DTAB, AOT and water. Click here to View figure |
DTAB+AOT/C10OH/H2O Systems
A series of pseudo phase equilibriums containing water, decanol and surfactants mixtures at various ratios was constructed. The resulting diagram were disclosed in Figure 3. Previously, in the DTAB/Alcohol/Water series, the result showed that system containing decanol was able to produce the largest region of liquid crystalline. Henceforth, decanol was chosen as the co-surfactant while constructing the following series of phase diagram.
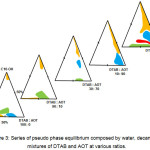 |
Figure 3: Series of pseudo phase equilibrium composed by water, decanol and mixtures of DTAB and AOT at various ratios. Click here to View figure |
From the DTAB/AOT/water system, it is found that DTAB and AOT mixtures at the ratio of 10:90, 70:30 and 90:10 had better compatibility on the formation of self-assembled aggregates since their aqueous form was able to exhibit various self-assembled aggregates at different water content. Thus, the surfactants mixtures at these ratios were used to construct a series of pseudo phase equilibrium containing decanol and water.
Optical Pattern
The optical patterns of the hexagonal liquid crystalline containing various alcohols were shown in Figure 4. In terms of normal micelles, the association structure generally was not affected by the alcohol chain length as well. Similarly, this region was able to solubilize approximately 10% of any alcohol. However, this generalization did not applicable to the butanol system. Interestingly, in the butanol system, the normal and reversed micelle regions were found to be joined together. This is probably due to its partial solubility in water which also explained the formation of the exceptionally large region of the isotropic dispersion instead of the formation of lamellar bilayer like the rest of the systems. Similar observation was found in the sodium carprylate/alcohol/H2O systems where the normal and reversed micelles regions were found to be conjoined together when water-soluble alcohols namely ethanol, methanol, propanol and butanol were used6.
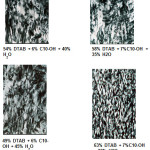 |
Figure 4: Optical patterns of hexagonal liquid crystals containing DTAB, alcohols and water Click here to View figure |
The results indicated the formation of inverted micelles was closely related to the chain length of alcohol where the significant effect was the solubility of DTAB in the alcohol. As the carbon chain length of the alcohol increased from butanol to decanol, the solubility of DTAB was dropped from 64% to 13% while no inverted micelles were found in dodecanol and hexadecanol system. This had clearly showed that the formation of inverted micelles by DTAB was restricted by the chain length of the alcohol. This implied that DTAB can only form inverted micelles with alcohol whose chain length were shorted than DTAB’s hydrophobe length. It appeared as if the extra carbon chain attached at the dodecanol and hexadecanol were too large for the DTAB’s hydrophobe to accommodate. Consequently, their presence in the system ruptured the inverted micelles structures. Besides that, the results also implied that the formation of lamellar liquid crystalline was also affected by the carbon chain length of the alcohol. Initially, the neat phase was not found in the butanol system. It began to appear in systems with alcohol containing at least six carbon atoms. With the increase in alcohol chain length from six carbon atoms to ten carbon atoms, the region’s area expanded. However, the region shrunk significantly when decanol was replaced with dodecanol and hexadecanol respectively. Though the chain length of the alcohol impacted the formation of lamellar liquid crystalline, however, it did not affect the optical pattern of the neat phase which was shown in Figure 5.
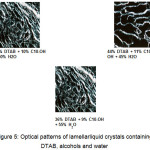 |
Figure 5: Optical patterns of lamellarliquid crystals containing DTAB, alcohols and water Click here to View figure |
Conclusion
As a summary, the phase behavior of DTAB in water and in the presence of various chain length alcohols was study. From the phase equilibrium constructed, it was concluded that DTAB was able to from four distinctive association structures in the presence of water and short chain alcohol with alky chain length containing four to twelve carbon atoms. However, the formation of the association structures namely normal and reversed micelle, hexagonal and lamellar liquid crystalline was strongly independent on the length of the alcohol alkyl chain. Furthermore, the phase behavior of aqueous DTAB in the presence of decanol and AOT was investigated as well. The presence of AOT in the system was able to enhance the formation of the reversed micelle, in the meantime, its presence also suppressed the formation of normal micelle. The formations of lamellar liquid crystalline and hexagonal liquid crystalline were strongly dependent on the AOT composition. At higher AOT composition, hexagonal liquid crystalline was more likely to form while at lower AOT composition, the system favoured the formation of lamellar liquid crystalline.
References
- McBain, J.W.; Martin, J.T. J. Am. Chem. Soc., 1914. 105, 957
CrossRef - Mukerjee, P.; Mysels, K.J. Critical micelle concentration of surfactants systems, National Standard data References Series, 1971, 36, US National Bureau of Standards
- Rosen, M.J. Surfactant and interfacial phenomenon, Wiley-Interscience, New York, 1971, 1
- Myers, D. Surfactant science and technology, 2nd Edition, VCH Publishers, Inc New York, 1992
- Tadros, T.F. Applied surfactants: Principles and application, Weinheim:Wiley-VCH Verlag GmbH & Co., KGaA, 2005
- Ekwall, P. Advances in Liquid crystals, G.H. Brown, Vol 1, Acdemic Press, New York, 1975
- Hamdan, S.; Laili, C.R. Oriental J. Chem, 2106, 32(4), 2073-2078
- Clint, J.H. J. Chem. Soc., Faraday Trans. I, 1975, 71:1327-1331
CrossRef - Scamehorn, J.F. Phenomenon in mixed surfactant systems. ACS Symposium Series No 311, Washington DC, 1986
- Nagaran, R. Micellization of binary surfactant systems in Mixed surfactant systems (P.M. Holland & D.N. Rubingh Editors), 65th Colloid and Surfactant Chemistry Symposium, ACS, Washington DC, 1992
- Hamdan, S. Laili, C.R.; Anuar, K. J. Disp. Sci. Technol., 1994, 15(2), 147-164
- Hall-Manning, T.J.; Holland, G.H.; Rennie, G.; Revell, P.; Hines, J.; Barratt, M.D.; Basketter, D.A. Food and Chemical Toxicology, 1998, 36(3), 233-238
CrossRef - Khan, S.A.; Marques, E.F. Current Opinion in Colloid & Interface Science, 2000, 4, 402-410
CrossRef - Raman, I.A.B.; Hamdan, S.; Tiddy, G.J.T (2003). Adv. Colloid Interface Sci., 2003, 106, 109-127.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









