Inhibition Effect of 2,2’-Bipyridyl on the Corrosion of Austenitic Stainless Steel in 0.5M H2SO4
Amel Gharbi1, 2, Abdelaziz Himour1, Sihem Abderrahmane1 and Karima Abderrahim1
and Karima Abderrahim1
1Surface Engineering Laboratory (L.I.S), Badji Mokhtar University, B.P.12-23000, Annaba, Algeria.
2Research Center In industrial Technology CRTI, P.O.Box 64, Cheraga 16014 Algiers, Algeria.
Corresponding Author E-mail: abderrahmanesihem@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/340134
The corrosion inhibition of AISI309 austenitic stainless steel by 2,2'-Bipyridyl in 0.5M H2SO4 at 298K was studied using the mass loss’ method, the potentiodynamic polarization (Tafel), the linear polarization (LRP) and the electrochemical impedance spectroscopy (EIS). The results showed a mixed inhibition mode and an increase in the charge transfer resistance, due to inhibitor molecules’ adsorption at steel surface. This latter obeys to Langmuir isotherm. The observation by scanning electron microscopy (SEM) and the analysis by energy dispersion spectrometry (EDS) confirm an inhibitor film’s presence. The calculated inhibition efficiencies are in accordance with 87.78% maximum value.
KEYWORDS:AISI309; 2,2'-Bipyridyl; Corrosion; Inhibition; EIS; Tafel
Download this article as:| Copy the following to cite this article: Gharbi A, Himour A, Abderrahmane S, Abderrahim K. Inhibition Effect of 2,2’-Bipyridyl on the Corrosion of Austenitic Stainless Steel in 0.5M H2SO4. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Gharbi A, Himour A, Abderrahmane S, Abderrahim K. Inhibition Effect of 2,2’-Bipyridyl on the Corrosion of Austenitic Stainless Steel in 0.5M H2SO4. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41652 |
Introduction
Austenitic stainless steels are widely used as building materials in the following fields: medical, automotive, food1, steam power stations2, piping systems, chemical factories and heat exchanger equipment3. Their resistance to corrosion is due to passive film formation of chromium oxide on the surface. However, these steels are sensitive to corrosion in certain acidic media4, including sulfuric acid and hydrochloric acid which are widely used for stripping, cleaning and descaling industrial processes5, 6.
The organic inhibitors’ use is a practical, economical and effective method of protecting materials against corrosion7. The heterocyclic organic compounds containing Nitrogen are considered in the literature as mild steel’s efficient corrosion inhibitors in acidic media8. Generally, the adsorption mechanism of these compounds is made by heteroatoms such as nitrogen, oxygen and sulfur9,10 and depends on inhibitor’s physic-chemical properties, electrolyte’s chemical composition and metal nature11,12.
Among the heterocyclic organic compounds, pyridine and its derivatives are effective inhibitors against mild steels’ corrosion in hydrochloric acid13-20. Moreover, pyridine plays a very important role in biological activity by its presence in natural and pharmaceutical products; generally, its derivatives possess various pharmacological properties13, 21. However, few studies were carried out on pyridine and its derivatives as stainless steels’ corrosion inhibitors in H2SO422, 23.
Organic compounds containing a single pyridine ring are the most used, whereas those containing several rings are rarely used, so it is necessary to study the steel inhibition by these compounds with pyridine several rings which efficiency is important.
In this work, the main objective is to study the corrosion inhibition of AISI309 austenitic stainless steel by 2,2′-Bipyridyl in 0.5M H2SO4, this inhibitor contains two pyridine rings. The corrosion behavior was pursued by the weight loss method, potentiodynamic polarization (Tafel), electrochemical impedance spectroscopy (EIS) and the samples’ surface was characterized by Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS).
Experimental
Materials and media
The studied steel is AISI309 austenitic stainless steel whose chemical composition, established by spark spectroscopy, is shown in Table 1.
Table 1: Chemical composition of AISI309 austenitic stainless steel (wt. %)
|
C |
Si |
Mn |
P |
S |
Al |
Cr |
Ni |
V |
|
0.4 |
1.58 |
0.74 |
0.019 |
0.012 |
0.28 |
23.94 |
14.23 |
0.08 |
The observation by light microscopy of the Nikon ECLIPSE LV 100ND shows an austenitic structure with precipitates at grain boundaries (Fig.1). The diffractogram X recorded on a diffractometer BRUKER D8, adjusted by Maud software (Fig.2), identifies the following phases: an austenitic phase Feγ at 86% and the rest of M23C6 and M7C3 carbides.
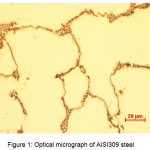 |
Figure 1: Optical micrograph of AISI309 steel Click here to View figure |
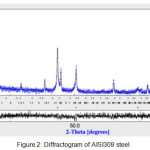 |
Figure 2: Diffractogram of AISI309 steel Click here to View figure |
The experimental inhibitor is 2, 2’-Bipyridyl, supplied by the company Sigma-Aldrich and whose chemical structure is shown in Fig.3. The studied medium is 0.5 M H2SO4 without and with the concentrations 10-6, 10-5, and 10-4M of 2,2′-Bipyridyl.
 |
Figure 3: Chemical structure of 2, 2’-Bipyridyl Click here to View figure |
Gravimetric Study
The gravimetric measurements were carried out on samples with the dimensions 1.2 cm x 1.35 cm x 0.7 cm in 50 ml of 0.5M H2SO4 without and with inhibitor’s different concentrations. After 2 hours immersion at 298 K, the samples were rinsed with distilled water and acetone and then dried and weighed using KERN ALS 220-4N analytical balance of precision ± 0.1 mg.
Electrochemical Measurements
Electrochemical measurements were carried out using Gamry interface 1000 potentiostat/ galvanostat, associated with Gamry Framwork software and equipped with a conventional electrochemical cell with three electrodes: saturated calomel electrode (SCE), platinum counter electrode and AISI 309 austenitic stainless steel working electrode. The latter is embedded in an epoxy resin delimiting 1cm2 flat working surface, polished successively with silicon carbide (SiC) abrasive papers with different particle sizes: 600, 800, 1000 and 1200 and finally with 3μ diamond paste. Afterwards, it is washed with distilled water, degreased with acetone, then washed again with distilled water and finally dried under a dry air stream.
The electrochemical measurements were made after one hour immersion at 298 K: (a) the polarization curves (Tafel) are plotted by scanning the potential range (-600 to +970) mV vs. SCE at 1mV s-1 rate; (b) polarization resistance (Rp) was measured from the obtained polarization curves at ±10 mV relative to corrosion potential; and (c) electrochemical impedance spectroscopy’s measurements (EIS) were carried out in the frequency range from 100 kHz to 10 mHz with 10 mV signal amplitude.
Surface Analysis by MEB-EDS
The observation and analysis of samples surface were carried out, after 72 hours immersion in 0.5M H2SO4 without and with 2,2′-Bipyridyl, by Zeiss EVO MA25 scanning electron microscope, related to OXFORD X-MaxN energy dispersive spectroscopy (EDS).
Results and Discussion
Mass Loss Measurement
Mass loss measurements of AISI309 stainless steel in 0.5M H2SO4 without and with different concentrations of 2,2′-Bipyridyl, after 2 hours immersion at 298K, were evaluated by the corrosion rate CR and the inhibition efficiency EWL(%), calculated by the following equations24.
CR = ΔW / At (1)
Where:
ΔW: mass loss;
A: sample surface (cm2);
t: immersion time (h).
EWL(%) = [(CR0 – CR) / CR0] x 100 (2)
Where CR0 and CR are respectively the corrosion rates (mg cm-2 h-1), without and with inhibitor addition in 0.5M H2SO4. The calculated values of corrosion rate (CR) and inhibition efficiency (EWL %) are shown in Table 2.
Table 2: Corrosion rate (CR) Values and inhibition efficiency EWL(%) obtained from gravimetric measurements.
|
Concentration of inhibitor (M) |
CR (mg cm-2 h-1) |
EWL (%) |
|
Blank |
0.2150 |
– |
|
10-6 |
0.0745 |
65.34 |
|
10-5 |
0.0575 |
73.25 |
|
10-4 |
0.0358 |
83.34 |
The results (Table 2) show that the inhibitor concentration’s increase decreases the corrosion rate and consequently raises the inhibition efficiency which reaches 83.34% maximum value at 10-4M (Fig.4). This increase is attributed to the adsorption of 2,2’-Bipyridyl molecules on steel surface leading to a protective film formation25.
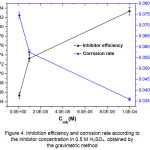 |
Figure 4: Inhibition efficiency and corrosion rate according to the inhibitor concentration in 0.5 M H2SO4, obtained by the gravimetric method Click here to View figure |
Measurements of electrochemical impedance spectroscopy
The electrochemical impedance spectra of AISI309 steel at 0.5M H2SO4 in Nyquist representation, plotted after 1 hour immersion without and with 2,2′-Bipyridyl, at the concentrations(10-6,10-5et 10-4M) are presented in Fig.5(a). Without 2,2′-Bipyridyl, the impedance spectrum is composed of two loops: a semicircular high frequency (HF) capacitive loop attributed to charge transfer reaction26,27 and an inductive loop at low frequencies (LF) attributed either to relaxation process due to Fe SO4 species’ adsorption or the inhibitor species on metal surface28, and / or at passive film’s re-dissolution29. With 2,2’-Bipyridyl, the impedance spectra are composed of a single capacitive loop indicating that the corrosion process is controlled by charge transfer phenomenon11. The inductive loop’s disappearance with 2,2′-Bipyridyl addition results from the protective film formation on steel surface30.
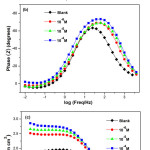 |
Figure 5: Impedance diagrams of AISI309 steel in 0.5M H2SO4 at different concentrations of 2,2′-Bipyridyl in representation of Nyquist (a) and Bode according to the phase angle (b) and the modulus (c) Click here to View figure |
The Nyquist curves with 2,2’-Bipyridyl at different concentrations are wider than that without inhibitor, their diameters increase with the inhibitor concentration increasing, thereby improving the inhibition efficiency31,32.
The obtained capacitive half-loops are not perfect; this may be attributed to the frequency dispersion effect, resulting from roughness and inhomogeneity of the electrode surface33. Therefore, instead of modeling the double layer by Cdl capacitance, it is preferable to use a constant phase element (CPE) whose impedance is described by the expression34.
ZCPE = Q-1 (j ω)-n (3)
Where:
Q is the CPE constant;
ω is the angular frequency (in rad s-1);
j2= -1 is the imaginary number.
The exponent n can characterize different physical phenomena such as surface inhomogeneities resulting from its roughness, inhibitor adsorption and porous layer’s formation35; its values vary from 0 to -1 and characterize the CPE properties36,37.
In Bode’s representation, the phase angle (Fig.5(b)) increases with the inhibitor at different concentrations compared to that without 2,2′-Bipyridyl, this increase is due to inhibitor film’s formation on metallic surface and ǀZǀ modulus’ increase (Fig.5(c)) indicates good inhibitor performance38. The equivalent circuits deduced from the impedance curves in representation of Nyquist without and with inhibitor are shown respectively in Fig.6(a) and Fig.6(b).
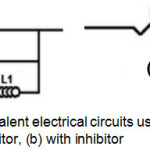 |
Figure 6: Models of equivalent electrical circuits used for the impedances’ analysis: (a) without inhibitor, (b) with inhibitor Click here to View figure |
Without inhibitor, the equivalent circuit is constituted of an electrolyte resistance (Rs), a constant phase element (CPE), a charge transfer resistance Rct, an inductive resistance (RL) and an inductance L. With inhibitor, the equivalent circuit is composed of an electrolyte resistance (Rs), a constant phase element (CPE) and a charge transfer resistance Rct. From this electrical model, a parametric adjustment was made (Fig.7) showing that both experimental and simulated spectra are well correlated with an adequacy coefficient χ2 of the order 23×10-3.
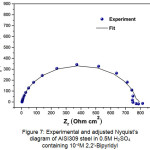 |
Figure 7: Experimental and adjusted Nyquist’s diagram of AISI309 steel in 0.5M H2SO4 containing 10-4M 2,2′-Bipyridyl Click here to View figure |
The values of various electrochemical parameters, deduced from the impedance spectra’s parametric adjustment, are reported in Table 3. The corrosion’s inhibition efficiencies are calculated from Rct values according to the following equation39
ERT(%) = [(Rct – Rct0) / Rct] x100 (4)
Where Rct0 and Rct are respectively steel charge transfer resistances’ values without and with inhibitor addition.
Table 3: Electrochemical impedance parameters for AISI309 in 0.5M H2SO4 without and with addition of 2, 2’-Bipyridyl various concentrations and the corresponding inhibition efficiency
|
Concentration of inhibitor (M) |
Rs (Ω cm2) |
Rct (Ω cm2) |
CPE (µF cm-2) |
N |
RL (Ω cm2) |
L (H cm2) |
ERt (%) |
|
Blank |
2.17 |
94.39 |
749.4 |
0.868 |
289.2 |
551 |
– |
|
10-6 |
3.31 |
303.6 |
202.7 |
0.894 |
– |
– |
68.90 |
|
10-5 |
3.42 |
424.8 |
179.4 |
0.875 |
– |
– |
77.78 |
|
10-4 |
3.83 |
773 |
111. 5 |
0.902 |
– |
– |
87.78 |
The values of these parameters show that: Rct value rises with the inhibitor concentration increase; this is attributed to a protective film’s formation at metal / solution interface, which enhances the inhibition efficiency39. The CPE values’ reduction can result from the increase of the inhibitor film’s thickness formed on metal surface may be due to 2,2′-Bipyridyl molecules’ adsorption at metal / solution interface40.
The maximum Rct (773Ω cm2) and the minimum CPE (111. 5µF cm-2) are reached at the 10-4M concentration of 2,2′-Bipyridyl.
The inhibition efficiency, calculated from Equation (4), rises according to 2,2′-Bipyridyl’s concentration’s increase and reaches 87.78% maximum value at 10-4M which is higher than that obtained by Ghazoui et al19. at 10-3M imidazopyridine derivative.
Open Circuit Potential
Figure 8 shows the variation of the AISI309 steel’s open-circuit potential EOCP without and with 10-4M 2,2′-Bipyridyl during 1 h of immersion.
Without inhibitor, the open-circuit potential tends to stabilize at -395mV / SCE, with inhibitor we note the EOCP displacement towards more positive values which is due to the inhibitor’s film formation.
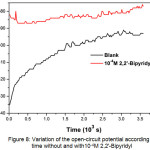 |
Figure 8: Variation of the open-circuit potential according to time without and with10-4M 2,2′-Bipyridyl Click here to View figure |
Potentiodynamic (Tafel) polarization
Figure 9 shows the samples’ polarization curves (Tafel) in 0.5M H2SO4 without and with 2,2′-Bipyridyl at different concentrations after 1 hour of immersion at 298 K. The curves have the same general shape with an anodic passivation plateau in the potential range between -0.2 and +0.8 V vs. SCE. The inhibitor addition reduces the anodic and cathodic current’s densities, shifts the corrosion potential to noble values and varies the Tafel’s slope values. The βc change indicates 2,2′-Bipyridyl influence on the cathodic reaction’s kinetics. The variation of the βa anode branch’s values may be due to the inhibitory molecules’ adsorption on metal surface.
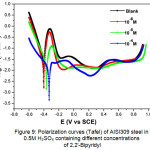 |
Figure 9: Polarization curves (Tafel) of AISI309 steel in 0.5M H2SO4 containing different concentrations of 2,2′-Bipyridyl Click here to View figure |
Generally, the inhibitor compound’s classification depends on its influence on the shifting of the corrosion potential (dEcorr.) compared to its value without inhibitor (Ecorr). If dEcorr > 85mV, the inhibitor is considered as an anodic or cathodic type, whereas if dEcorr< 85mV, it is considered to be a mixed type25, 41. In this study, dEcorr = +73 mV to the anodic direction indicating that the 2,2′-Bipyridyl inhibitor acts as a mixed inhibitor with mainly an anodic effect. This behavior is due to 2,2′-Bipyridyl molecules’ adsorption which blocks the cathodic and anodic active sites by reducing the cathodic hydrogen release’s rate and the slowing down of metal anodic dissolution’s reactions7.
In Table 4, the electrochemical parameters are grouped, derived from the polarization curves (Tafel), such as the corrosion potential (Ecorr), the slope of cathodic Tafel (βc) and anodic (βa), the corrosion current density (icorr) and the polarization resistance (Rp) measured from the (LRP) method; Without inhibitor, icorr = 55.57 μA cm-2 and Rp = 94.28 Ω cm-2, they reach respectively 7.68 μA cm-2 and 753 Ω cm-2 at 10-4M of 2,2′-Bipyridyl.
The inhibition efficiencies EI(%) and ERp(%) were calculated from the polarization resistance and the corrosion current density without and with inhibitor according to Eqs. (5) (6)42.
Ei(%) =[ (icorr – icorr(inhi)) / icorr ] x 100 (5)
Where:
icorr: corrosion current density with inhibitor;
icorr(inh): corrosion current density without inhibitor.
ERP(%) = [ (Rp(inh) – Rp) / Rp(inh) ] x 100 (6)
Where:
Rp: polarization resistance without inhibitor;
Rp(inh): polarization resistance with inhibitor.
The best inhibition efficiency Ei (86.17%) obtained at 10-4M 2,2′-Bipyridyl is the same found by Bin Xu et al16. at 3mM N-Bis (2-pyridylmethyl) aniline in 1MHCl.
We note a concordance of the obtained values Ei (86.17%) and ERP (87.47%).
Table 4: Steel electrochemical parameters sat various concentrations of 2, 2’-Bipyridyl in 0.5M H2SO4 and calculated inhibition efficiencies
|
Concentration of inhibitor (M) |
Ecorr, (mV vs SCE) |
icorr, (µA cm-2) |
–βc (mV dec-1) |
βa (mV dec-1) |
Rp (Ω cm2) |
Ei (%) |
ERp (%) |
|
Blank |
-403.7 |
55.57 |
104.7 |
56.4 |
94.28 |
– |
– |
|
10-6 |
-401.4 |
17.83 |
86.0 |
37 |
325.3 |
67.91 |
71.01 |
|
10-5 |
-366.9 |
14.89 |
83.7 |
52 |
467.4 |
73.20 |
79.82 |
|
10-4 |
-330.6 |
7.68 |
124.8 |
79.4 |
753 |
86.17 |
87.47 |
Adsorption Isotherm
In general, the molecules’ adsorption process on metallic surface is reflected by water molecules’ replacement adsorbed by organic molecules according to the following reaction43.
Org(sol) + xH2O(ads) ↔ Org(ads) + xH2O(sol) (7)
Where:
Org(sol): dissolved organic molecules in solution;
H2O(sol) : water molecules in solution;
Org(ads) : organic molecules adsorbed at metallic surface;
H2O(ads) : water molecules adsorbed at metallic surface;
x: water molecules’ number substituted by organic molecules.
The adsorption mechanism as well as the inhibitors behavior can be explained by thermodynamic interpretation of the adsorption’s isotherms, established from potentiodynamic polarization measurements.
The adsorption isotherm is represented by Cinh/ θ graph according to Cinhi (Fig.10).
Where, the metallic surface coverage (θ) by the adsorbed atoms is calculated using the following equation 44.
θ = Ei (%) / 100 (8)
EI (%): inhibition efficiency calculated depending on corrosion current densities.
The best adjustment gives a characteristic straight line of Langmuir isotherm whose regression coefficient’s value (R2 = 0.9998) is close to unity. According to this model, the surface coverage θ is linked to the adsorption equilibrium constant Kads by the equation41.
Cinh / θ = 1/ Kads + Cinh (9)
Where:
Cinh : inhibitor concentration;
Kads can be calculated from the fit line’s intersection on Cinh / θ axis.
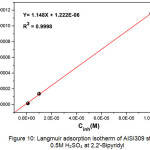 |
Figure 10: Langmuir adsorption isotherm of AISI309 steel in 0.5M H2SO4 at 2,2′-Bipyridyl different concentrations Click here to View figure |
The standard free energy of adsorption (ΔG0ads) is obtained according to the following equation45:
ΔG = -RT ln(55.5 Kads) (10)
Where:
R: universal gas constant (8.314 J mol-1 K-1);
T: absolute temperature;
The value 55, 5 is the water concentration in solution (mol L-1).
The high value of Kads = 0.818 x 106 M-1 indicates 2,2’-Bipyridyl‘s strong adsorption on steel surface giving 86.17% à 10-4M inhibition efficiency.
For ΔGads ≤ -20 kJ mol-1, the organic molecules are physisorbed on metallic surface, whereas for ΔGads ≥ -40 kJ mol-1, the inhibitory molecules are chemisorbed on metallic surface by charge sharing or transfer46. In this study, the calculated value of ΔGads is -43.68 kJ mol-1, thus 2,2’-Bipyridyl adsorption is spontaneous and chemisorption.
Scanning electron microscopy
Figure 11 shows samples surface’s micrographs, obtained by MEB after 72 hours of immersion in 0.5M H2SO4 without and with 2,2′-Bipyridyl 10-4M; Without inhibitor (Fig. 11(a)), the surface is rough and strongly corroded; With inhibitor (Fig. 11(b)), it appears to be less attacked and has a rather smooth aspect; the spectra (EDS) corresponding to the 02 states are presented in Fig. 12(a),(b). By comparison, the obtained elements’ atomic percentages are reported in Table 5; the inhibited case demonstrates a peak’s appearance at 4.09% nitrogen, a carbon percentage’s increase from 9.46% to 20.25%, a decrease in iron percentage from 58.27% to 49.50% and the absence of sulfur peak. These results confirm the inhibitor molecules’ adsorption on samples surface.
 |
Figure 11: Steel MEB images after 72h immersion in 0.5M H2SO4, (a) without inhibitor, (b) with 10-4M 2,2’-Bipyridyl Click here to View figure |
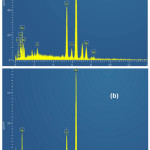 |
Figure 12: Steel EDS spectra after 72h immersion in 0.5M H2SO4: (a) without inhibitor; (b) with 10-4M 2,2′-Bipyridyl Click here to View figure |
Table 5: Elements’ atomic percentages obtained from the EDS spectra
|
Media |
Fe |
Cr |
C |
O |
S |
N |
Ni |
|
0.5M H2SO4 |
58.27 |
17.44 |
9.46 |
4.41 |
0.89 |
0.00 |
9.54 |
|
0.5M H2SO4 +10-4 M 2,2’-Bipyridyl |
49.50 |
15.14 |
20.25 |
3.89 |
0.00 |
4.09 |
6.94 |
Conclusion
The corrosion inhibition of AISI309 austenitic stainless steel by 2,2′-Bipyridyl in 0.5M H2SO4 was studied by gravimetric, potentiometric polarization (Tafel), electrochemical impedance spectroscopy, and micrographic analyzes by MEB and EDS. The obtained results show that:
2,2′-Bipyridyl presents good inhibitory properties, its efficiency is 83.34% at 10-4M.
2,2′-Bipyridyl acts as a mixed inhibitor with a mainly anodic effect.
The inhibitor concentration increase causes a raise of charge transfer resistance.
2,2′-Bipyridyl adsorption obeys to Langmuir isotherm and Gibbs energy ΔGads = -43.68 kJ mol-1 indicates a lot more chemical than physical adsorption.
SEM observation and EDS analysis confirm an inhibitor film’s formation on metallic surface.
References
- Pandya, S.; Ramakrishna, K.S.; Annamalai,A. R.; Upadhyaya, A. Mater. Sci. Eng. A 2012, 556, 271-277
CrossRef - BAI, G.; Lu, S.; Li, D.; Li, Y. Corros. Sci. 2016, 108, 111-124
CrossRef - Ibrahim, M. A. M.; Abd El Rehim, S. S; Hamza, M. M. Mater. Chem. Phys. 2009, 115, 80-85
CrossRef - Mehdipour, M.; Naderi, R.; Markhali, B. P. Prog. Org. Coat. 2014, 77, 1761-1767
CrossRef - Yadav, M.; Kumar, S.; Purkait, T.; Olasunkanmi, L.O.; Bahadur, I.; Ebenso, E.E. J. Mol. Liq. 2016, 213, 122-138
CrossRef - Verma, C.; Ebenso, E.E.; Bahadur, I.; Obot, I.B.; Quraishi, M. A. J. Mol. Liq. 2015, 212, 209-218
CrossRef - Keles, H.; Keles, M. Res. Chem. Intermed. 2014, 40, 193-209
CrossRef - Karthik, G.; Sundaravadivelu, M.; Rajkumar, P. Res. Chem. Intermed. 2015, 41, 1543-1558
CrossRef - Gobara, M.; Baraka, A.; Zaghloul, B. Res. Chem. Intermed. 2015, 41, 7245-7261
CrossRef - Kosari, A.; Moayed, M.H.; Davoodi, A.; Parvizi, R.; Momeni, M.; Eshghi, H.; Moradi, H. Corros. Sci. 2014, 78, 138-150
CrossRef - John, S.; Jeevana, R.; Aravindakshan, K.K.; Joseph, A. Egypt. J. Pet. 2016
- Yadav, M.; Gope, L.; Sarkar, T.K. Res. Chem. Intermed. 2016, 42, 2641-2660
CrossRef - Ansari, K.R.; Quraishi, M.A.; Singh, A. Meas. 2015, 76, 136-147
CrossRef - Yüce, A.O; Telli, E.; Mert, B.D.; Kardaş, G.; Yazıcı, B. J. Mol. Liq. 2016, 218, 384-392
CrossRef - Yıldız, R. ; Döner, A. ; Doğan, Dehri, T. İ. Corros. Sci. 2014, 82, 125-132
CrossRef - Xu, B.; Ji, Y.; Zhang, X.; Jin, X.; Yang, W.; Chen, Y. J. Taiwan Inst. Chem. Eng. 2016, 59, 526-535
CrossRef - Sayin, K.; Jafari, H.; Mohsenifar, F. J. Taiwan Inst. Chem. Eng. 2016, 68, 431-439
CrossRef - Belkaid, S.; Tebbji, K.; Mansri, A.; Chetouani, A. ; Hammouti, B. Res. Chem. Intermed. 2012, 38, 2309-2325
CrossRef - Ghazoui, A.; Saddik, R.; Hammouti, B.; Zarrouk, A.; Benchat, N.; Guenbour, M.; Al-Deyab, S.S.; I. Warad, Res. Chem. Intermed. 2013, 39, 2369-2377
CrossRef - Karthik, R.; Vimaladevi, G.; Chen, S.M.; Elangovan, A.; Jeyaprabha, B.; P. Prakash, Int. J. Electrochem. Sci. 2015, 10, 4666-4681
- Ansari, K.R.; Quraishi, M.A.; Singh, A. J. Ind. Eng. Chem. 2015, 25, 89-98
CrossRef - Hamza, M.M.; Abd El Rehim, S.S.; Ibrahim, M. A. M. Arab. J. Chem. 2013, 6, 413-422
CrossRef - Obaid, A. Y.; Ganash, A. A.; Qusti, A.H.; Elroby, S.A.; Hermas, A. A. Arab. J. Chem. 2013
- Amoozadeh, S. M.; Mahdavian, M.; J. Mater. Eng. Perform. 2015, 24, 2464-2472
CrossRef - El Faydy, M.; Galai, M.; El Assyry, A.; Tazouti, A.; Touir, R.; Lakhrissi, B.; Ebn Touhami, M.; Zarrouk, A. J. Mol. Liq. 2016, 219, 396-404
CrossRef - Jina, Z.H.; Gea, H.H.; Lina, W.W.; Zongb, Y.W.; Liub, S.J.; Shi, J.M. Appl. Surf. Sci. 2014, 322, 47-56
CrossRef - Sin, H. L.Y.; Umeda, M.; Shironita, S.; Abdul Rahim, A.; Saad, B. Res. Chem. Intermed. 2016, 1-16
- Abd El-Lateef, H. M. Res. Chem. Intermed. 2016, 42, 3219-3240Khadiri, A.; Saddik, R.; Bekkouche, K.; Aouniti, Hammouti, A. B.; Benchat, N.; Bouachrine, M.; Solmaz, R. J. Taiwan Inst. Chem. Eng. 2016, 58, 552-564
- Solmaz, R.; şahin, E. A.; Doner, A.; KardaŞ, G.; Corros. Sci. 2011, 53, 3231-3240
CrossRef - Lebrini, M.; Bentiss, F.; Chihib, N. E.; Jama, C.; Hornez, J. P.; Lagrenée, M. Corros. Sci., 2008, 50, 2914-2918
CrossRef - Prabakaran, M.; Kim, S.H.; Hemapriya, V.; Chung, I. M. Res. Chem. Intermed., 2016, 42, 3703-3719
CrossRef - Lebrini, M.; Lagrenée, M.; Vezin, H.; Traisnel, M.; Bentiss, F. Corros. Sci., 2007, 49, 2254-2269
CrossRef - Jiang, B.; Jiang, S.L.; Liu, X.; Ma, A. L.; Zheng, Y.G. J. Mater. Eng. Perform. 2015, 24, 4797-4808
CrossRef - Shabani-Nooshabadi, M.; Ghandchi, M.S. J. Ind. Eng. Chem. 2015, 31, 231-237
CrossRef - RameshKumar, S.; Danaee, I.; RashvandAvei, M.; Vijayan, M. J. Mol. Liq. 2015, 212, 168-186
CrossRef - Yilmaz, N.; Fitoz, A.; Ergun, Ü.; Emregül, K.C. Corros. Sci. 2016, 111, 110-120Pavithra, M. K.; Venkatesha, T. V.; Punith Kumar, M. K.; Anantha, N. S. Res. Chem. Intermed. 2016, 42, 2409-2428
- Raja, P.B.; Rahim, A.A.; Osman, H.; Awang, K. Int. J. Miner. Metall. Mater. 2011, 18, 413-418
CrossRef - Obot, I. B.; Madhankumar, A. Mater. Chem. Phys. 2016, 177, 266-275
CrossRef - Kumari, P.; Shetty, P.; Rao, S. A.; Sunil, D. Trans. Indian. Inst. Met. 2016.
- Anbarasi, K. Electrochemical and Corrosion Inhibition Studies of Cucurbita Maxima. Orient. J. Chem., 2016, 32, 2139-2145.
CrossRef - Saranya, J.; Sounthari, P.; Kiruthika, A.; Saranya, G.; Yuvarani, S.; Parameswari, K.; Chitra, S. Experimental and quantum chemical studies on the inhibition potential of some quinoxaline derivatives for mild steel in acid media. Orient. J. Chem, 2014, 30, 1719-1736.
CrossRef - Baskar, R.; Gopiraman, M.; Kesavan, D.; Subramanian, K.; Gopalakrishnan, S. J. Mater. Eng. Perform. 2015, 24, 2847-2856
CrossRef - Sasikala, T.; Parameswari, K.; Chitra, S.; Synthesis and Corrosion Inhibition Study of Benzothiazepine Derivatives on Mild Steel in Acid Medium. Orient. J. Chem. 2016, 32, 1215-1222.
CrossRef - Liu, X.; Okafor, P. C.; Jiang, B.; Hu, H.; Zheng, Y. J. Mater. Eng. Perform. 2015, 24, 3599-3606.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









