Hydration of Strontium-doped Monocalcium Aluminate
Anti Kolonial Prodjosantoso , Septiani, Maximus Pranjoto Utomo and Kun Sri Budiasih
, Septiani, Maximus Pranjoto Utomo and Kun Sri Budiasih
Department of Chemistry, Yogyakarta State University, Yogyakarta, DIY 55281 Indonesia.
Corresponding Author E-mail: prodjosantoso@uny.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340148
Monocalcium aluminate (CA) is the major hydraulic phase in the calcium aluminate cements (CAC). Solid solution of Ca1-xSrxAl2O4may be formed if the cement raw materials are impure with Sr mineral. This study aims to understand the hydration of Ca1-xSrxAl2O4. The nature, crystallinity and microstructure of hydrated phases were identified using scanning electron microscopy (SEM-EDX), thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC), X-ray diffraction (XRD) and infrared spectroscopy (IR Spectroscopy). There is a strong indication that the hydration produces a mixture of Ca1-xSrxAl2(OH)12 and AH3.
KEYWORDS:Monocalcium Aluminate; Hydration; Ca1-xSrxAl2(OH)12; AH3
Download this article as:| Copy the following to cite this article: Prodjosantoso A. K, Septiani S, Utomo M. P, Budiasih K. S. Hydration of Strontium-doped Monocalcium Aluminate. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Prodjosantoso A. K, Septiani S, Utomo M. P, Budiasih K. S. Hydration of Strontium-doped Monocalcium Aluminate. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42516 |
Introduction
Calcium aluminate cements (CAC) have alumina contents varying from about 38% to 90% w/w, in the form of monocalcium aluminate (CaAl2O4 or CA). Second phases include belite, corundum, dodecacalcium hepta-aluminate, ferrite pleocroite, gehlenite, monocalcium dialuminate, and wüstite. The CA is the major hydraulic reacting with water to form a series of calcium aluminate hydrates, which vary with temperature and time.1–4
At low temperatures (<20ºC), the main crystalline hydrate formed is CAH10, and an amorphous phase is formed in considerable amounts.5–7 This amorphous phase is usually taken to be alumina gel, but Payne and Sharp have argued that it must be one containing a calcium aluminate hydrate of unknown composition since no CH [Ca(OH)2] or calcium rich aluminate hydrates are simultaneously formed to maintain a chemical balance.8 At higher temperatures, C2AH8 is formed as well as or instead of CAH10. Both of these hydrates are hexagonal in morphology and thermodynamically metastable. Above 28ºC, they convert rapidly into C3AH6which has a cubic structure, and gibbsite [Al(OH)3 or AH3].7 The reactions are schematically summarized below.
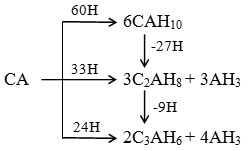
Although the presence of major hydrates, CAH10[CaAl2(OH)8(H2O)6], C3AH6[Ca3Al 2(OH)12] and AH3 [Al(OH)3], can be detected by X-ray diffraction (XRD), the peaks are not readily suitable for quantitative analysis. Preferred orientation can occur with hexagonal phases such as CAH10, and also the hydrates may not be entirely crystalline, especially at the early stages of hydration. Also there are phases, which are amorphous by nature, e.g. the gel phases, and the presence of these will not be detected by XRD.6,7 Therefore, the use of thermal analysis techniques is very important in the study of CA as it gives information as to the hydrate phases present in the early stages of hydration, when this may not be possible from XRD.9
The morphology and mechanism of crystallization of the gel phase has previously been studied by TEM.10 Although there have been some reports about conversion reactions products in high alumina and calcium aluminate cements, but there is lack of information about the exact microstructure and growth of these phases.11
Solid solution of Ca1-xSrxAl2O4 formed if the cement raw materials are impure with Sr mineral is recently investigated. Attempts have been taken to give a clear understanding of Ca1-xSrxAl2O4 hydration, by applying different methods, such as SEM/EDX, TGA, DSC, XRD, and IR spectrometry.
Experimental
Polycrystalline samples of Ca1-xSrxAl2O4 were prepared from mixtures of analytical grade CaCO3 (Univar 99%), SrCO3 (Merck pa.), and Al(NO3)3.9H2O (Aldrich 98%). For each composition the appropriate stoichiometric mixture was very thoroughly ground in an agate mortar and pestle for several minutes. The mixtures were then treated using method described by Prodjosantoso.12,13
Hydrated samples were prepared by adding H2O to the finely powdered oxides in the mole ratio of 100:1.14 The mixtures were stirred for 2 weeks under a nitrogen atmosphere at ambient temperature. The reactions were terminated by addition of acetone, and the solids were collected by filtration and then dried under a stream of dry nitrogen. The resulting materials were then characterized using Scanning Electron Microscope (Hitachi SU 3500), Thermogravimetry Analyzer (Mettler Toledo TGA-DSC 1), Powder X-Ray Diffractometer (Shimadzu XRD–6000 ), and Fourier Transform Infrared Spectrophotometer (Shimadzu IR Prestige-21).
Results and Discussions
The hydration of Ca1-xSrxAl2O4was undertaken at intermediate temperature ie. at between 28 to 36°C for 14 days. The morphologies of the unhydrated and hydrated Ca1-xMxAl2O4 samples were inspected using SEM. In general, the samples consist of non-descript shaped crystals with sizes varying in the range 0.01 to 0.1 mm. A selected SEM micrograph of the samples is displayed in Figure 1.
 |
Figure 1: SEM micrograph of unhydrated (a) and hydrated Ca0.5Sr0.5Al2O4 (b). Click here to View figure |
EDA has been used to check the atomic composition of the samples. For the seven Sr-doped samples the Sr contents derived from the EDA analysis are systematically high, however the observed Ca:Sr:Al ratios are in reasonable agreement with the expected values (Table 1.). EDA spectra for unhydrated and hydrated Ca0.5Sr0.5Al2O4 are given in Figure 2. SEM and EDA methods were succeed to qualitative and quantitative elemental analysis but were not recognized the formation of AH3, C2AH8, CAH10 and Ca1-xSrxAl2(OH)12 in the samples.
Table 1: Atomic ratio of Ca1-xSrxAl2O4 (e.s.d. + 0.005).
| Ca1-xSrxAl2O4, x = | Atoms | ||
| Ca | Sr | Al | |
| 0 | 1.01 | 0 | 1.99 |
| 0.125 | 0.90 | 0.16 | 1.94 |
| 0.25 | 0.75 | 0.30 | 1.95 |
| 0.5 | 0.46 | 0.55 | 1.98 |
| 0.75 | 0.19 | 0.79 | 2.02 |
| 0.875 | 0.14 | 0.86 | 2.00 |
| 1 | 0 | 1.06 | 1.94 |
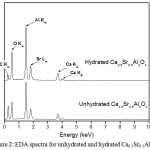 |
Figure 2: EDA spectra for unhydrated and hydrated Ca0.5Sr0.5Al2O4. Click here to View figure |
The occurrence of AH3, C2AH8, CAH10 and Ca1-xSrxAl2(OH)12 in the hydrated samples were then confirmed by TGA-DSC thermograph, however evidences indicate the formation of AH3, and Ca1-xSrxAl2(OH)12. The TGA-DSC plot of hydrated Ca0.5Sr0.5Al2O4 is depicted in Figure 3., while the thermal decompositions of hydrated Ca1-xSrxAl2O4 are listed in Table 2.
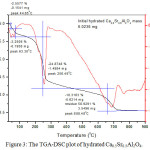 |
Figure 3: The TGA-DSC plot of hydrated Ca0.5Sr0.5Al2O4. Click here to View figure |
Table 2: Thermal decomposition of hydrated Ca1-xSrxAl2O4
| Compounds | Thermal decomposition | Residue | ||
| (%) | (mg) | (°C) | ||
| Hydrated CaAl2O4(5.6762 mg) | -3.3750 | -0.1916 | 60.76 | 60.4499 %3.4313 mg |
| -22.0846 | -1.2536 | 250.95 | ||
| -14.1532 | -0.8034 | 702.02 | ||
| Hydrated Ca0.75Sr0.25Al2O4(5.9988 mg) | -20.6942 | -1.2414 | 281.10 | 61.6984 %3.7012 mg |
| -17.6154 | -1.0567 | 709.86 | ||
| Hydrated Ca0.5Sr0.5Al2O4(6.0236 mg) | -2.5577 | -0.1541 | 44.65 | 58.9291 %3.5496 mg |
| -3.2509 | -0.1958 | 63.30 | ||
| -24.8748 | -1.4984 | 256.49 | ||
| -10.3163 | -0.6214 | 688.48 | ||
| Hydrated Ca0.1Sr0.9Al2O4(5.9957 mg) | -5.0147 | -0.3007 | 53.00 | 58.4119 %3.5022 mg |
| -3.9761 | -0.2384 | 69.47 | ||
| -29.8422 | -1.7892 | 253.79 | ||
| -2.6644 | -0.1597 | 680.43 | ||
| Hydrated SrAl2O4(5.9433 mg) | -21.9251 | -1.3031 | 281.99 | 68.1024 %4.0475 mg |
| -10.0172 | -0.5953 | 805.64 | ||
Some samples indicate stages weight loss at temperature below 80°C indicating evaporation of acetone used to terminate dehydration processes, and trapped in the solid samples. The rest of the stages, in general, consist of a two main stage weight loss. The first one, in the range of 250−300°C, is attributed to the decomposition of Al(OH)3, which is probably in the form of gibbsite, bayerite and nordstandite. Gibbsite, bayerite and nordstandite are dehydrated at 300, 275, and 280°C, respectively, and are decomposed to gamma-, eta-, and eta-Al2O3, respectively. Broad peak at about 500 to 600°C indicates the coarse gibbsite and bayerite, which are partially decomposed first to boehmite at 210 and 225°C, respectively, and then to gamma-Al2O3 at 510°C and eta-Al2O3 at 505°C.
The characteristic feature of amorphous Al(OH)3 phase such as the gradually changing and step-less weight loss curve and the broad DSC endothermic structure between 200°C and 500°Cis confirmed. This supports the conclusion that the hydrated samples contain amorphous Al(OH)3 as major species. The weak endotherms at about 250°C and 420°C represent amorphous Al(OH)3 to amorphous Al2O3 decomposition.
It is difficult to estimate the relative amounts of amorphous Al(OH)3 in the sample based on the DSC endothermic peaks. When a sample is a mixture of different chemical species, and the weight change is much slower due to, for example, release of strongly adsorbed water, than the temperature ramping rate, its DSC curve does not necessarily show their endo- and exo-therms at the same temperatures and can be weakened and broadened. A DSC peak height is not often related to the amount of the weight loss. If the weight decreases linearly with increasing temperature, the DSC curve will look flat although there is a weight loss. However, the TGA data seems to suggests that Al(OH)3 is a major species.
The main loss of water occurred at around 300°C indicates the presence of Ca1-xSrxAl2(OH)12. The water loss of up to 350°C equals to approximately 5H2O. The total water loss up to 600°C (28 wt.%) is consistent with the presence of 6 waters in Ca1-xSrxAl2(OH)12, similar toC3AH6.15 These crystalline powders also show sharp endothermic peaks and stepwise weight losses at 300 to 600°C. Structural analyses suggested the following decomposition processes for Ca1-xSrxAl2(OH)12 in air:
Ca1-xSrxAl2(OH)12 →Ca1-xSrxAl2O6.2H2O + 4H2O (262-326°C)
Ca1-xSrxAl2O6.2H2O →Ca1-xSrxAl2O6.H2O + H2O (400°C)
Ca1-xSrxAl2O6. H2O →Ca1-xSrxAl2O6 + H2O (>600°C)
There is no evidence of mass loses between 350°C to 550°C indicating the absence of the Ca(OH)2 and or Sr(OH)2 decompositions.1617
The crystalline phases present in the unhydrated and hydrated Ca1-xMxAl2O4samples were investigated using XRD method. The X-ray diffraction pattern peak height and 2q position measurements of unhydrated and hydrated Ca1-xMxAl2O4 are graphically shown in Figure 4. The different of both patterns indicates that all unhydrated samples were hydrated completely producing different phases.
Similar to C3AH6, the crystalline Ca1-xSrxAl2(OH)12 is thermodynamically stable in the temperature range from 20 to 250°C,18,19 allowing this hydroxide be analyzed using XRD method. The XRD pattern of the samples prepared from Ca1-xMxAl2O4 in the present study shows the crystalline Ca1-xSrxAl2(OH)12 indicated by a strong reflection at 2θ at about 17.27°. Minor peaks were observed, which could be assigned to Al(OH)3crystalline phases.
FTIR has been used to collect evidences supporting the presence of Ca1-xSrxAl2(OH)12 and AH3 in the hydrated Ca1-xSrxAl2O4. The FTIR spectra of selected hydrated samples are depicted in Figure 6, and the IR frequencies are listed in Table 3.
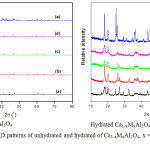 |
Figure 4: Powder XRD patterns of unhydrated and hydrated of Ca1-xMxAl2O4, x = 0 (a), 0.25 (b), 0.5 (c), 0.9 (d), and 1 (e). Click here to View figure |
The formation of Ca1-xSrxAl2(OH)12 could take place at the presence of a low concentration of OH–, e.g. pH = 7-8. In basic systems, the solubility of Al(OH)3 is quite large.
High solubility of the ionic species favors a large amount of hydrated ions releasing for participation in the homogeneous formation reaction; this also eliminates the chance for the occurrence of impurities. On the other hand, highly crystalline Ca1-xSrxAl2(OH)12 can be formed in wider ranges of input concentrations. In the corresponding IR spectrum in Figure 5., the absorption band at ca. 3600 cm-1assigned to-OH stretching vibration of the lattice -OH in Ca1-xSrxAl2(OH)12, and the bands at ca. 3450 and 1600 cm-1assigned to-OH stretching and bending vibrations, respectively, in adsorbed water were also observed.20,21
The absorptions at about 850 cm-1 and 913 cm-1 are the characteristic absorption of the Al-O stretching bond.22 The absorption at 1030 cm-1 is assigned to Al-OH bond. The weak absorption at 666 cm-1 is the absorption of CaO, Sr-O bonds or the combination of both of the compounds Ca1-xSrxAl2(OH)12. The complete IR frequencies of hydrated Ca1-xSrxAl2O4 is displayed in Table 3.
 |
Figure 5: The FTIR spectra of selected hydrated Ca1-xSrxAl2O4 samples. Click here to View figure |
Table 3: The IR frequencies of hydrated Ca1-xSrxAl2O4.
| Type of bond | Frequencies (cm-1) | ||||
| Hydrated CaAl2O4 | Hydrated Ca0.75Sr0.25Al2O4 | Hydrated Ca0.5Sr0.5Al2O4 | Hydrated Ca0.1Sr0.9Al2O4 | Hydrated SrAl2O4 | |
| Al-O stretching | 852.59 | 852.82 | 856.93 | 857.95 | 857.74 |
| 913.17 | 913.91 | 915.35 | 913.96 | 913.36 | |
| Al-OH bending | 1022.86 | 1024.20 | 1024.99 | 1020.81 | 1022.06 |
| Al-OH stretching | 3525.73 | 3526.14 | 3525.63 | 3523.33 | 3523.98 |
| Ca-O, Sr-O stretching | 663.38 | 666.07 | 664.80 | 666.31 | 667.74 |
| O-H bending | 1637.61 | 1641.65 | 1638.63 | 1632.65 | 1634.99 |
| O-H stretching | 3469.78 | 3469.13 | 3468.14 | 3465.63 | 3461.86 |
Conclusions
The hydration of Ca1-xSrxAl2O4 solid solution for 14 days at ambient temperature produces a mixture of crystalline Ca1-xSrxAl2(OH)12 and very poorly crystalline AH3. Crystalline phases of Ca(OH)2 and Sr(OH)2 are not observed in the hydrated Ca1-xSrxAl2(OH)12.
References
- Stinnessen, I.; Buhr, A.; Kockegey-lorenz, R.; Racher, R., Tech. Pap., 2001.
- Scrivener, K., Advanced Concrete Technology, 2003, 1–31.
- Scrivener, K. L.; Cabiron, J. L.; Letourneux, R., Cem. Concr. Res., 1999, 29, 1215–1223. DOI: 10.1016/S0008-8846(99)00103-9
Cross0Ref - Pöllmann, H.; Kaden, R., Fentiman CH, Mangabhai RJ Scrivener KL, 2014, 18–21.
- Antonovič, V.; Keriene, J.; Boris, R.; Aleknevičius, M., Procedia Engineering, 2013, 57, 99–106.
Cross0Ref - Wang, P.; Xu, L., Procedia Eng., 2017, 27, 253–260.
Cross0Ref - Klaus, S. R.; Neubauer, J.; Goetz-Neunhoeffer, F., Cem. Concr. Res., 2013, 43, 62–69. DOI: 10.1016/j.cemconres.2012.09.005
Cross0Ref - Payne, D. R.; Sharp, J. H., The Microstructure and Chemistry of Cement and Concrete, 1989.
- Bushnell-Watson, S. M.; Sharp, J. H., Cem. Concr. Res., 1990, 20, 677.
Cross0Ref - Richardson, I. G.; Skibsted, J.; Black, L., Kirkpatrick, R. J., Adv. Cem. Res., 2010, 22, 233–248. DOI: 10.1680/adcr.2010.22.4.233
Cross0Ref - Rashid, S.; Turrillas, X.; Thermochim. Acta, 1997, 302, 25–34.
Cross0Ref - Prodjosantoso, A., Kennedy, B., Mater. Res. Bull., 2003, 38, 79–87. DOI: 10.1016/S0025-5408(02)01009-7
Cross0Ref - Prodjosantoso, A. K.; Kennedy, B. J.; J. Solid State Chem., 2002, 168, 229–236. DOI: 10.1006/jssc.2002.9715
Cross0Ref - Prodjosantoso, A. K.; Kennedy, B. J.; Hunter, B. A.; Cem. Concr. Res., 2002, 32, 647–655. DOI: 10.1016/S0008-8846(01)00737-2
Cross0Ref - Dilnesa, B. Z.; Lothenbach, B.; Renaudin, G.; Wichser, A.; Kulik, D., Cem. Concr. Res., 2014. 59, 96–111. DOI: 10.1016/j.cemconres.2014.02.001
Cross0Ref - Kim, T.; Olek, J.; Transp. Res. Rec. J. Transp. Res. Board, 2012, 2290, 10–18. DOI: 10.3141/2290-02
Cross0Ref - Tangy, A.; Pulidindi, I. N.; Gedanken, A.; Energy & Fuels, 2016, 30, 3151–3160. DOI: 10.1021/acs.energyfuels.6b00256
Cross0Ref - Ball, M. C.; Cem. Concr. Res., 1976, 6, 419–420.
Cross0Ref - Plank, J.; Zhang-Preβe, M.; Ivleva, N. P.; Niessner, R., Constr. Build. Mater., 2016, 122, 426–434. DOI: 10.1016/j.conbuildmat.2016.06.042
Cross0Ref - Kolesov, B. A.; Geiger, C. A., Am. Mineral., 2005, 90, 1335–1341. DOI: 10.2138/am.2005.1622
Cross0Ref - Saikia, B. J.; Parthasarathy, G., J. Mod. Phys., 2010, 1, 206–210. DOI: 10.4236/jmp.2010.14031
Cross0Ref - Torréns-Martín, D.; Fernández-Carrasco, L.; Martínez-Ramírez, S., Cem. Concr. Res., 2013, 47, 43–50. DOI: 10.1016/j.cemconres.2013.01.015.
Cross0Ref

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









