Fabrication of Sugar Palm Fiber/Andisol Soil Composites for Iron(III) ion Removal in Aqueous Solution
Pranoto1,2 , Abu Masykur1,2, Nurul Fatimah1 and Santika Kunti Prabawani1
, Abu Masykur1,2, Nurul Fatimah1 and Santika Kunti Prabawani1
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Sebelas Maret University, Jl. Ir. Sutami 36A Surakarta, Indonesia, 57126.
2Analytical and Environmental Chemistry Research Group, Sebelas Maret University, Jl. Ir. Sutami 36A Surakarta, Indonesia, 57126.
Corresponding Author E-mail: pakpranotomipa@staff.uns.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340137
The rapid development of industrial sector gave not only positive impact on national income but also a negative impact on the environment. Iron (III) heavy metal ion is one of harmful metal ion for biotic creatures and human. In order to control the metal ion in wastewater, adsorption method was introduced. Andisol soil (AndS) containing allophane and sugar palm fiber (SPf) having cellulose are potential materials utilized as an adsorbent for iron metal ion removal in aqueous solution. In this study, the AndS/SPf composite was successfully fabricated and characterized as well as optimized as an adsorbent. The adsorption process was performed using a batch method by varying pH condition, calcination temperature, AndS:SPf ratio and contact time. This study obtained that the optimum pH by 5, calcination temperature by 100°C, AndS:SPf ratio by 1:3 and contact time by 45 minutes. This research also studied the isotherm adsorption, i.e. Langmuir and Freundlich model and found that the adsorption followed Freundlich model.
KEYWORDS:Adsorption; Andisol Soil; Batch Method; Iron Metal Ion; Sugar Palm Fiber
Download this article as:| Copy the following to cite this article: Pranoto P, Masykur A, Fatimah N, Prabawani S. K. Fabrication of Sugar Palm Fiber/Andisol Soil Composites for Iron(III) ion Removal in Aqueous Solution. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Pranoto P, Masykur A, Fatimah N, Prabawani S. K. Fabrication of Sugar Palm Fiber/Andisol Soil Composites for Iron(III) ion Removal in Aqueous Solution. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=43042 |
Introduction
Heavy metal ions contained in industrial wastewater is increasing great attention among researchers and governments since their toxic nature and harmful for biotic creatures as well as for human sustainability. It is generated from industrial activities such as mining, tanneries, paper industries, pesticides, batteries, etc. [1]. Various heavy metal ions reported as potentially hazardous to human are chromium, iron, copper, cobalt, nickel, cadmium, lead, arsenic, zinc, mercury, vanadium and others harmful metal ions [2, 3]. In order to reduce the harmful contents, especially metal ions, in wastewater, some approaches were introduced viz. precipitation, electrochemical, biological treatment, coagulation-flocculation, physic-chemical treatment, adsorption and others reviewed by Ngah et al. [4] Adsorption method is the simplest, low operational cost, and low energy requirement method often used by researcher for wastewater treatment [5, 6]. The adsorbent plays an important role in the adsorption process. Thus it is necessary to choose the suitable adsorbent. Moreover, the low cost and easy availability are also important parameters for choosing the adsorbent type.
Andisol soil (AndS) contained allophane is potential to use as an adsorbent to maintain heavy metal ion in aqueous solution already studied by Pazos et al. [7]. Andisol soil is widely available in Indonesia since it rich of Volcanoes Mountains and produced from weathering of volcanic rocks. In our previous study, we have successfully utilized the andisol soil composed with another mineral and biomaterial for chromium ion removal showing good adsorption ability toward the metal ion [8].
Agriculture waste product, today, is currently used as an adsorbent for wastewater containing heavy metals treatments due to their low cost, high efficiency, minimizing chemicals, possibly metal recover and many others [3]. Cellulose is the most abundant biomaterial in nature and could be extracted from agriculture waste product. Cellulose was often used as biosorbent to adsorb such heavy metal ions since cellulose provides many advantages such as low cost, easy preparation, available in nature and simple operation [9-11]. One of the agriculture waste products having a high concentration of cellulose is sugar palm (Arengga pinnata). According to Sanyang et al. [12], sugar palm contains a high concentration of cellulose, approximately 60%. Since it high content of cellulose, sugar palm fiber (SPf) is potential for adsorbent. In this work, we composed the andisol soil containing allophane with sugar palm fiber to produce adsorbents for iron metal ion removal.
Combination of andisol soil containing allophane and cellulose derived from sugar palm is a new approach in our group. In this study, we varied the raw materials ratio in composing of AndS/SPf composites in order to find the best composition chosen from the high adsorption capacity. Furthermore, the pH and contact time were also optimized for this study.
Materials and Methods
Materials
Andisol soil (AndS) was obtained from Lawu Mountain, East Java-Indonesia and prepared to a fine powder (150 mesh). Sugar Palm fiber (SPf) was collected from Klaten, Central of Java-Indonesia and mashed into 150 mesh particle size. The chemicals like 1000 mg.L-1 Fe(III) solution, HCl 37%, HNO3 67%, sodium hydroxide were commercially purchased from Merck. Distilled water was obtained from UNS laboratory.
General Method
Both fine powders of AndS and SPf were chemically treated before fabrication with sodium hydroxide solution. About 50 g of AndS was treated with 250 mL of 3M NaOH for 5 h at 70°C. Afterward, the treated AndS was filtered and washed with distilled water several times for neutralizing. Finally, the AndS was dried for 4 h at 105°C. About 50 g of SPf was soaked in 500 mL of 0.1M NaOH for 20 minutes at 80°C. Then, the treated SPf was filtered, washed with distilled water and dried for 24 h at 50°C.
The composites were fabricated via sonically assisted synthesis at room temperature. The raw material with adjusted ratio AndS:SPf 1:0 (F1), 0:1 (F2), 1:1 (F3), 1:2 (F4) and 2:1 (F5) w/w was mixed, stirred and sonicated for 1 h in a distilled water medium. After that, the mixture was filtered, dried at 105 °C for 4 h and crushed into 150 mesh fine powder.
The raw materials and composites were characterized using FTIR (IR Prestige-21 Shimadzhu) and XRD (Shimadzhu 6000). FTIR characterization was recorded with KBr powder in range 4000-400 cm-1, resolution 2 cm-1, and the number of the scan by 50 times. XRD characterization was recorded using Cu Kα.
Adsorption Procedures
There were four parameters optimized in this study, i.e. composite ratio, pH condition, calcination temperature and adsorption contact time. Approximately, 0.05 g of composites was added into a beaker containing 10 mL of adjusted pH Fe (6 mg/L) solution. Then, the mixture was shaken for desire contact time, filtered immediately and the filtrate was analyzed using Atomic Adsorption Spectroscopy (AAS) model Perkin Elmer Analyst 700.
In this research was also studied the isotherm adsorption after obtaining the optimum condition. About 0.05 g composite was added to 10 mL of Fe (2, 4, 6, 8 and 10 mg/L) solution which they made from dissolving Fe liquor in HNO3 solution. The adsorption process was taken at optimum contact time. Two models were used to study the isotherm adsorption, such as Langmuir and Freundlich models following Eq. 1 and 2, respectively.
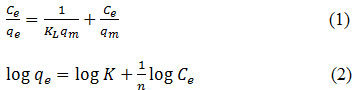
where Ce (mg/L)is a concentration of Fe solution at equilibrium, qe (mg/g) and qm (mg/g) are adsorption capacity at equilibrium and maximum, KL and K is Langmuir and Freundlich constant, n is constant.
Results and Discussion
Materials Characterization
Functional group analysis of each component and its composite was characterized using FTIR. Fig. 1 reveals the IR spectrum of each raw materials and their characteristic after chemical treatment. As seen in Fig. 1a, the AndS has characteristic vibration bands at around 463 cm-1, 550 cm-1, 1007 cm-1, 1600 cm-1, and 3410 cm-1 assigned for Si-O-Si bending, Al-O-Si bending, Si-O stretching, H2O, and O-H stretching vibration. Both AndS and treated AndS have same characteristic vibration band. However, the differences between their spectrums were the intensity. After treatment with acid, the intensity of OH stretching vibration was lower than AndS. Moreover, the Si-O stretching vibration was found sharper than origin one. It was probably due to the impurities were removed by the chemical treatment. It also found by Saputra et al. (2017). Fig. 1b shows the IR spectrum of SPf and treated SPf. Sugar palm fiber contains cellulose, hemicellulose, and lignin. Before treatment, the IR spectrum of SPf showed the presence of carbonyl group appeared in 1730 cm-1 corresponding to lignin structure. However, after treatment with a basic solution, the absorbance band of carbonyl group vanished. It indicates that the lignin content, as well as hemicellulose, was removed.
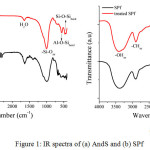 |
Figure 1: IR spectra of (a) AndS and (b) SPf Click here to View figure |
Fig. 2 exposes the IR spectra of each treated raw materials and the AndS/SPf composite. It was clearly seen that all of the characteristic peaks of both andisol soil and sugar palm fiber appeared in IR spectra of AndS/SPf composite. It indicated that homogeneous distribution of materials in the composite system. The peak at 3410 cm-1 assigned to hydroxyl group both for AndS and SPf in the composite system. In the IR spectra of AndS/SPf was also revealed peaks at 2902 cm-1, 1007 cm-1, 553 cm-1, and 462 cm-1 corresponding to C-H stretching, overlapping Si-O and C-O stretching, Si-O-Al bending and Si-O-Si bending vibration, respectively.
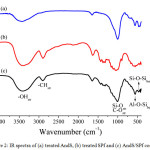 |
Figure 2: IR spectra of (a) treated AndS, (b) treated SPf and (c) AndS/SPf composite Click here to View figure |
In this study, the XRD characterization of treated materials and composite was performed to evaluate the components of each material and composite which they were compared to the JCPDS standard. The XRD patterns of AndS, SPf and AndS/SPf composite shown in Fig. 3. As compared to the JCPDS, the andisol soil of Lawu Mountain contains allophane phase (2θ = 27.6°, JCPDS no 02-0039), feldspar (2θ = 21.6°, JCPDS no 84-0710), and gibbsite (2θ = 19.6°, JCPDS no 07-0324). The SPf diffractogram also revealed that the main component is cellulose proven by the presence of peaks at 2θ 15.76 and 22.72° similar to JCPDS no 50-2241. All of these characteristic diffractogram peaks appeared in composite XRD diffractogram. This analysis matched with IR analysis.
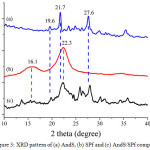 |
Figure 3: XRD pattern of (a) AndS, (b) SPf and (c) AndS/SPf composite Click here to View figure |
Adsorption Study
The pH condition is the important parameters in the adsorption process. Since its necessity, this research has studied the effect of pH on adsorption capacity as seen in Fig. 4. In this research, the pH was only performed in acid condition due to at basic condition, the iron metal ions was easily to settle [13]. At low pH condition, the adsorption capacity of AndS/SPf composite was relatively lower approximately 0.29 mg/g (pH= 2). In this case, the presence of proton contributes to increase the competition with the metal ions. Moreover, the H+ was able to protonate the adsorbent surface leading to improve the positive charge causing the interaction between iron metal ions become weak. However, after the pH was increased, the adsorption capacity also enhanced until pH 5 having adsorption capacity by 0.83 mg/g. Based on the data, the best pH condition was pH 5.
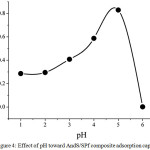 |
Figure 4: Effect of pH toward AndS/SPf composite adsorption capacity Click here to View figure |
Calcination temperature related to the characteristic of adsorbent. Fig. 5 shows the effect of calcination temperature on adsorption capacity. The aim of calcination is to remove the solvent or organic substances in the composite surface or in the cavities. In this research, the calcination temperature was started from 100°C until 400°C. As seen in Fig. 5, the adsorption capacity decreased by increasing the temperature calcination. It was probably due to the structure of cellulose in the composite was degraded by heating. Thus, the adsorption capacity at 400°C became lower, which is 0.79 mg/g. However, at 100°C of calcination temperature, it only reduced the water molecules entrapped either in composite surface or cavities. The optimum condition regarding calcination temperature was 100°C having adsorption capacity by 0.95 mg/g.
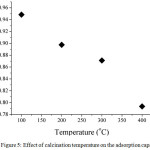 |
Figure 5: Effect of calcination temperature on the adsorption capacity Click here to View figure |
Optimization of the composite ratio aimed to determine the best composition of raw materials in the composite fabrication. There are five composites optimized, namely F1, F2, F3, F4 and F5 with AndS:SPf ratio by 1:0, 0:1, 1:1, 1:2 and 2:1, respectively. As presented in Fig. 6, the AndS (F1) has lower adsorption capacity compared to other composites, which is 0.21 mg/g. However, after compositing with SPf, the adsorption capacity was dramatically increased to 0.95 mg/g, 0.77 mg/g, and 1.05 mg/g, respectively for F3, F4 and F5. Based on the data, it can be concluded that the optimum ratio of AndS/SPf composite was F5 with AndS:SPf ratio by 1:3. The presence of SPf plays important role in iron metal removal in aqueous solution since it has a high concentration of cellulose capable to adsorb the metal ions [9].
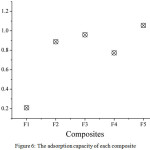 |
Figure 6: The adsorption capacity of each composite Click here to View figure |
Fig. 7 shows the adsorption capacity of iron metal ions adsorbed onto AndS/SPf composite influenced by adsorption contact time. In the initial time (15 minutes) the adsorption capacity was 1.01 mg/g. Afterward, the adsorption capacity increased by long contact time until reached equilibrium state at 45 minutes having adsorption capacity by 1.06 mg/g. Then, the adsorption capacity decreased dramatically to 1.03 mg/g. The decreasing of adsorption capacity due to the desorption process of iron metal ion after reaching optimal condition. The weak interaction of iron metal to the adsorbent active site could be a possible mechanism on this issue. To evaluate the adsorption mechanism, two adsorption isotherm models were approached.
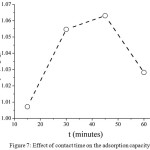 |
Figure 7: Effect of contact time on the adsorption capacity Click here to View figure |
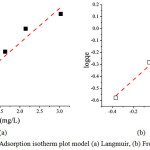 |
Figure 8: Adsorption isotherm plot model (a) Langmuir, (b) Freundlich Click here to View figure |
As described before that the adsorption of iron metal was weakened after reaching an equilibrium state. The mechanism of adsorption of iron metal ion was studied by two model which often used by researchers, namely Langmuir and Freundlich models. Fig. 8 and Fig. 9 reveal the Langmuir and Freundlich adsorption isotherm model, respectively. The fitting linier showed that the Freundlich model was suitable for the adsorption having R2 by 0.99437 (see Table 1). It means that the adsorption mechanism was physisorption where the adsorbate form multilayer in the adsorbent active site. The monolayer adsorption capacity calculated from Langmuir equation was 3.31 mg/g. The adsorption of iron metal ion was categorized as favorable adsorption since the 1/n value was in range 0<1/n<1.
Conclusion
The AndS/SPf composite was successfully prepared and characterized in this study. The FTIR analysis made a good agreement with XRD data regarding the homogenous distribution of each element in composite fabrication. The AndS/SPf composite was used as an adsorbent for iron metal ion adsorption and found that it optimal to adsorb at pH 5. Moreover, the optimum calcination temperature, composite ratio and contact time were found at 100°C, AndS:SPf 1:3, and 45 minutes. The Adsorption mechanism followed Freundlich model with monolayer adsorption capacity calculated from Langmuir model by 3.31 mg/g.
Acknowledgements
Authors would like to thank all member of Analytical and Environmental Chemistry Research Group and Allophane Research group for all support in this research.
References
- Celik, A.; Demirbas, A. Energ. Source. 2005, 27, 1167–1177
CrossRef - Garg, U.K.; Kaur, M.P.; Garg, V.K.; Sud, D. J. Hazard. Mater. 2007, 140, 60–68
CrossRef - Sud, D.; Mahajan, G.; Kaur, M.P. Bioresource Technol. 2008, 99, 6017-6027.
CrossRef - Ngah, W.S.W.; Teong, L.S.; Hanafiah, M.A.K.M. Carbohyd. Polym. 2011, 83, 1446-1456.
CrossRef - Vafakhah, S.; Bahrololooma, M.E.; Bazarganlari, R.; Saeedikhani, M. J. Environ. Chem. Eng. 2014, 2, 356–361.
CrossRef - Qiu, G.; Xie, Q.; Liu, H.; Chen, T.; Xie, J.; Li, H. Appl. Clay Sci. 2015, 118, 107–115.
CrossRef - Pazos, M. T. F.; Rodriguez, B. G.; Munoz, J. C. N.; Estevez, M. A.; Sanjurjo, M. J. F.; Delgado, A. N.; Alvarez, E. Water Air Soil Pollut. 2013, 224, 1366.
CrossRef - Pranoto; Sajidan; Suprapto; A. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 176, 012022.
- Miretzky, P.; Cirelli, A.F. J. Hazard. Mater. 2010, 180, 1-19.
CrossRef - Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. J. Hazard. Mater. 2013, 263, 322-333.
CrossRef - Hegazi, H.A. HBRC J. 2013, 9, 276-282.
CrossRef - Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. J. Renew. Sustain. Energ. 2015, 54, 533-549.
CrossRef - Sajidu, S.M.I.; Persson, I.; Masamba, W.R.L.; Henry, E.M.T.; Kayambazinthu, D. Water SA. 2006, 32(4), 519-526.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









