Extraction of Insignificant Amounts of Phenols in Walnut (Juglans Regia) in Aqueous Samples
Hossainali Sheybani
Department of Agronomy, Varamin-Pishva Branch, Islamic Azad University.
Corresponding author E-mail: dr.sheybani@iauvaramin.ac
DOI : http://dx.doi.org/10.13005/ojc/340151
In recent years, emphasis has been placed on the use of natural materials in the control and treatment of various infections, as some chemically synthesized drugs have undesirable side effects. Beech forests play an important role in temperate in Golband area northern of Iran since they occupy infertile montane soils.Solid phase extraction of insignificant amounts of Phenols in Walnut (Juglans regia) in aqueous samples using carbon nanotubes and visible and ultraviolet spectrophotometry measurement in biologic samples is used in his study. These systems include two phases, aqueous donor phase and conjugated carbon nanotube acceptor phase. Experiments were performed in two steps, aqueous phase extraction and of Phenols in Walnut (Juglans regia) resorption using methanol acidic solvent and resorbed samples were provided to Vis-UV spectrophotometry for further analysis. This method is cheap, simple, fast, and compatible with most of instrumental analysis methods. Extraction parameters including resorbing organic solvent effect, pH of donor and acceptor phases, extraction duration, resorption duration, shaking time, volume of donor phases and surfactant effect were optimized and analysis and measurements were performed under optimized situation. Mentioned techniques had many advantages including; short extraction time, low consumption of organic solvents, removing the effect of prior experiments, low diagnosis threshold and high concentration factor. Concentration factor and diagnosis threshold were to be 51 and 9.51 for Phenols in Walnut (Juglans regia) . Linear domain and relative standard was 1.1153%.
KEYWORDS:Phenols in Walnut (Juglans Regia); Solid Phase Extraction; Carboxyl Conjugated Carbon Nanotubes; Spectrophotometry
Download this article as:| Copy the following to cite this article: Sheybani H. Extraction of Insignificant Amounts of Phenols in Walnut (Juglans Regia) in Aqueous Samples. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Sheybani H. Extraction of Insignificant Amounts of Phenols in Walnut (Juglans Regia) in Aqueous Samples. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42389 |
Introduction
Th e genus Juglans (family Juglandaceae) comprises several species and is widely distributed throughout the world. Green walnuts, shells, kernels and seeds, bark, and leaves are used in the pharmaceutical and cosmetic industries. Phenols in Walnut (Juglans regia) leaves are involved in many in processes and frequently are released into the environment through industrial discharges. Moreover nitrophenols and chlorophenols occur in the environment as degradation products of the organophos phorus and chlorinated phenoxyalkanoic acid pesticides, respectively. Anilines also occur in the environment as degradation products of the phenyl urea and dinitroaniline herbicides. Phenols are per sistent in the environment and toxic at the low mg/l level [1]. In the 80/778/EEC directive of the European Union it is stated that the maximum admissible concentration for each individual phenol in drinking water should not exceed 0.1 mg/ l [2]. Anilines are also of toxicological importance and the monitoring of their levels in environmental waters is Break important for the protection of health and the environment [3-16]. In this study, we are going to measure Phenols in Walnut (Juglans regia) in real samples by the interaction between nano absorbent and drug through UV-Visible spectrophotometer. The method is based on the interaction between the drug and nano absorbent. Parameters affecting the interaction and those affecting the measurement will be examined.
Experimental Section
Chemicals and Reagents
Standard solutions were prepared from Darmstadt, Germany of Merck, Method and dried for a week over phosphorus pentoxide in a vacuum desiccators before use. Carbon nanotubes were prepared from Merck [17]. All solutions were prepared with doubly distilled deionized water from Darmstadt, Germany of Merck. It was conditioned before use by suspending in 4 M nitric acid for 20 min, and then washed two times with water. One sites were intensively studied (insects collected, leaf phenolics determined and plant coverage measured) in Golband area northern of Iran. The study area is located on Parcel 206 Series 2, Jomand forestry plan in Golband forest. The average highest level of area1580 meters the, the average slope is 30 percent and the general direction is northeast .The rainy season, autumn, 633 mm with most rain and low rain seasons, spring rainfall is 134 mm. The average annual temperature is 16 degrees centigrade. The most cold month, January with temperatures 7°C and the hottest month of year is August with average temperature is 25.5 degrees Celsius. Along the northern slope of Alborz montains, 14 autochthonous beech populations Phenols in Walnut (Juglans regia) aged between 80 and 160 years were investigated. It was decided to select five locations along the distribution area of beech (in Hyrcanian zone) from Golband (Noshahr) and to establish three investigation stations in each regions (low, middle and high altitude of beech distribution range) to cover most of the geographical range of beech in Iran (Fig. 1). In each population beech twigs with dormant buds were sampled from 50 nonadjacent individuals (to avoid the sampling of related trees) chosen at random over a 3-4 ha area in a homogeneous environment.
 |
Figure 1: Distribution of studied regions Click here to View figure |
Synthesis of Functionalized Nanotubes with Amine
0.5233g of raw multi-walled carbon nanotubes was added to the solution of 1 to 3 (v) nitric acid and sulfuric acid. The resulting mixture was placed in an ultrasonic bath with a frequency of 40 KHz for 30 minutes, followed by a 24-hour mixing of the reflux. The resulting product was washed with distilled water until pH under the filter reached to about 7. The separated solid phase was dried for 12 hours at 60°C and under vacuum. COOH-MWCNT, generated in the second stage was mixed with ethylene diamine (20 ml) and was placed in an ultrasonic bath (40 kHz) at 60°C for 5 hours. The resulting mixture was stirred at 60°C for 24 hours, and the membrane of the resulting solid was removed by filtration of 0.22 µm polycarbonate powder and then it was washed with methanol without water. The resulting solid was dried overnight in a vacuum, and consequently MWCNT-NH 2, was obtained [8].
Instrumentation
Double beam ultraviolet-visible spectrophotometer, Model UV1700, in Razi Laboratory, University of Science and Research. These conditions are tabulated in. The pH measurements used by Sartorius model PB-11.
Carboxyl Conjugated Carbon Nanotubes Synthesis Method
0.5233 gram of multiwall raw carbon nanotubes is added to 4 to 3 nitic acid to sulfuric acid. The solution is kept in 11 kHz ultrasonic bath for 31 minutes, then it was refluxed in agitator for 31 hours. Obtained product was washed with distilled water until its pH reaches 9. Solid phases is separated and is dried in vacuum for 43 hours in 61 degrees centigrade.COOH-MWCNT, produced in the second step is mixed with 31 milliliters of ethylene di amine (mixed for 9 hours in 11 kHz ultrasonic bath). Obtained mixture was agitated for 31 hours at 61 degrees centigrade and 1.33 micron Millipore polycarbonate was separated in membrane filter and the solid product was washed with water free methanol.
Obtained solid product was dried in vacuum overnight so that MWCNT-COOH was produced [18-37].
Preparation of Method
In this study, new solid phase techniques are used to detect insignificant amounts of Phenols in Walnut (Juglans regia) in aqueous samples using carbon nanotubes and UV-visible spectrophotometry in biological samples. These techniques are a biphasic system in which donor phase is aqueous sample and acceptor phase are conjugated carbon nanotubes. Experiments were performed in two steps, aqueous phase extraction and of Phenols in Walnut (Juglans regia) resorption using methanol acidic solvent and resorbed samples were provided to Vis-UV spectrophotometry for further analysis. This method is cheap, simple, fast, and compatible with most of instrumental analysis methods. Extraction parameters including resorbing organic solvent effect, pH of donor and acceptor phases, extraction duration, resorption duration, shaking time, volume of donor phases and surfactant effect were optimized and analysis and measurements were performed under optimized situation.
pH effect on Phenols in Walnut (Juglans regia) extraction
To assess the effect of pH in Phenols in Walnut (Juglans regia) extraction, the following steps were performed initially. For each container, the desired tampon is added, since the goal is to determine appropriate pH. The lowest absorption is considered the best value.
50 ml of the drug with 500 pm concentration for Phenols in Walnut (Juglans regia) was taken. 1.15 g of carboxyl absorbent, 5 ml of buffer in 2-10 range were taken and added to a 50 ml balloon and delivered to volume with distillated deionized water. Then the balloon is shaked for 15 minutes, centrifuged for 15 minutes, and then passed through syringe filters and finally quantitative analysis of Phenols in Walnut (Juglans regia) took place.
Analytical Performance
After optimizing all effective parameters on absorption intensity, calibration curve of the method was drawn. For this purpose, 10 ml volumetric flask were filled with different concentrations of the medication. Then sodium choroid salt was added to 0.12 g Phenols in Walnut (Juglans regia) in percentage form and carboxyl conjugated carbon nanotube absorbent were added to the volumetric flask with optimum pH. Finally, it was delivered to volume by adding distillated deionized water. Then washing and … steps were performed. Finally absorption intensity of the solutions were recorded in laboratory temperature and calibration curve was drawn.
Results and Discussion
Assessment of results of SEM spectrum
In the figure below, SEM picture of carboxyl conjugated carbon nanotubes are depicted. For carboxyl nanotubes, particles with 200 nm size are produced (Figure 2).
 |
Figure 2: SEM picture of carbon nanotubes before absorption Click here to View figure |
Moreover, SEM picture after absorption shows placement of desired metal on carboxyl conjugated carbon nanotubes from which it is inferred that the width of the sheets is increased. As it is seen in the picture, carboxyl functional group is shown as more bright points on the surface of carbon nanotubes (Figure3).
 |
Figure 3: SEM picture of carbon nanotubes after absorption Click here to View figure |
Primary experiment: absorbent effect of Phenols in Walnut (Juglans regia) extraction
According to the charts, carboxyl conjugated carbon nanotubes showed better absorption. Consequently, carboxyl absorbent was selected for Phenols in Walnut (Juglans regia) extraction (Figure4).All spectrums were put together in 298 nm of wavelength. As a result, this wavelength was determined to be the optimum absorption wavelength.
pH Effect on Phenols in Walnut (Juglans Regia) Extraction
This chart shows that pH=6 is appropriate for protonating carbon nanotubes which is associated with higher Phenols in Walnut (Juglans regia) absorption on carboxyl conjugated carbon nanotubes. Thus, the best electrostatic situation for absorbent and the drug for surface attraction are present at pH=6.0
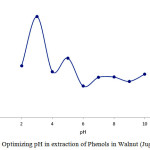 |
Figure 4: Optimizing pH in extraction of Phenols in Walnut (Juglans regia) Click here to View figure |
Absorbent amount in Phenols in Walnut (Juglans regia) extraction
Another parameter affecting absorption, is the amount of absorbent. 0.12 g was selected for Phenols in Walnut (Juglans regia) . Reported results indicate that din lower amount of absorbent, some substances may enter the solution since absorption may happen in drug maximum wave length (Figure5).
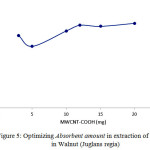 |
Figure 5: Optimizing Absorbent amount in extraction of Phenols in Walnut (Juglans regia) Click here to View figure |
Real Sample
In order to study the effects of sample matrix on the performance of the sorbent, the recovery results were examined using real-life sample spiked with the phenolic compounds at two different concentration levels. Phenols in Leaves of Juglans regia compounds sample was at 2–3 µg l−1 levels. After the SPE and derivatization step, an aliquot of final extraction was UV-Vis. The limits of detection using 20 ml of Phenols in Leaves of Juglans regia compounds sample were calculated based on a signal-to-noise ratio of 3 and were in the range of 15–120 ng l−1, using SPE- UV-Vis (Table 1).
Table 1: The extraction recoveries obtained for the studied Phenols in Walnut (Juglans regia) leaves at 20 ml sample in the range between 2 and 3 µg1−1 using MWCNT- COOH a.
| MWCNT- COOH | ||
| LOD (ppm) | %Recovery | RSD(%) |
| 14.4 | 70.4 | 3.5 |
| 15.8 | 80.2 | 5.8 |
| 12.8 | 88.3 | 4.9 |
| 15.3 | 70.7 | 8.6 |
| 19.1 | 65.9 | 9.4 |
a The relative standard deviations (RSD) between 3.5–9.4% (n = 4).
Conclusion
In the methodology of the conducted research and presented results in previous chapters, diffusing solid phase extraction technique and UV-vis spectrophotometer, precondensation and measurement of slight amounts of Phenols in Walnut (Juglans regia) in biological samples have been used. This study was intended to develop a high efficacy, selective, cheap and simple method for evaluating Phenols in Walnut (Juglans regia) amount in biological samples. Developing solid phase extraction method in the resent years, introduced the need for an effective absorbent.Thus, in this study, carboxyl conjugated carbon nanotubes were used to enhance the yield of Phenols in Walnut (Juglans regia) extraction. Effective parameter including pH, buffer type and concentration, amount of absorbent, type and volume of washing solvent, rate (time) of reaction, and salt effect were assessed. This method has good repeatability and a wide linear range (0.1-10 ppm) and proper condensation factor to determine Phenols in Walnut (Juglans regia) . It also as a good linear range, limit of detection of 6.6 ppb and high repeatability are other properties of this method.
References
- Taher M.A., Mostafavi A., Afzali D., Rezaeipour E., Bull. Korean Chem. Soc. 25 ,2004, 1125–1129.
CrossRef - Tuzen, M. ;Soylak, M. ; Citak, D. ; Ferreira, H.S. ; Korn M.G.A. ; Bezerra, M.A., J. Hazard. Mater.,2009,162, 1041-1048.
CrossRef - Moghimi A., Russian Journal of Physical Chemistry A,87(11) ( 2013) 1851–1858.
- Hagen, D.F.; Markell, C.G.; Schmitt, G.A. ;Anal.Chim. Acta1990,236,157-165.
CrossRef - Hart A.P., Dasgupta A., J. Forensic Sci. 42 ,1997, 693.
CrossRef - Heberer T., Stan H.J., Anal. Chim. Acta 341 ,1997, 21.
CrossRef - Nick K., Scholer H.F., Fresenius J. Anal. Chem. 343 ,1992, 304.
CrossRef - Dasgupta A., Jagannath C., Ther. Drug Monit. 21 ,1999, 238.
CrossRef - Haas R., Smidt T.C., Steinbach K., von Low E., Fresenius J. Anal. Chem. 359 ,1997,497.
CrossRef - Longo M., Cavallaro A., J. Chromatogr. A 753 ,1996, 91.
CrossRef - Lee H.B., J. Chromatogr. 457 , 1988, 267.
- Moghimi , A. ; Russian Journal of Physical Chemistry A,2013,87(7), 1203–1209.
- Moghimi A., Journal of Chemical Health Risks .2014, 4(2), 15–22.
- Moghimi A,.Aust J Basic &Appl Sci. 2012,6(3), 320-330.
- Schniepp, H.C.; Li, J.-L.; McAllister, M. J.;Sai, H.; Herrera-Alonso, M.; Adamson, D. H. ; Prud’homme, R. K. ; Car, R. ; Saville, D. A. ; Aksay, I. A. ,J. Phys. Chem. B, 2006, 110, 8535-8542.
CrossRef - Schilt, A.A.; Hoyle, W.C. ,Anal. Chem. 1964,41,344-349.
CrossRef - Borchart, L.G.; Butler, J.P. ;Anal. Chem. 1957,29,414-419.
CrossRef - De La Cruz, F. A.; Cowley, J. M. , Nature 1962, 196, 468-473.
CrossRef - Shuai Wang, B. ; Jon Chia, P.; Chua, L.; Hong Zhao, L.; Qi Png, R.; Sivaramakrishnan, S.; Wee, S. ; Ho, H. ; Adv. Mater. 2008, 20, 3440–3446
CrossRef - Karousis, N.; Sandanayaka,A.S.D.; Hasobe,T.;Economopoulos,S.P.;Sarantopouloua,E.; Tagmatarchis, N.,J. Mater. Chem., 2011, 21, 109-115.
CrossRef - Smith MB, March J. March’s advanced organic chemistry: reactions,mechanisms, and structure. New York: John Wiley & Sons Inc.; 2001, 1182–3.
- Mermoux M, Chabre Y, Rousseau A. FTIR and carbon-13 NMR study of graphite oxide. Carbon 1991,29(3),469–74.
CrossRef - Cataldo F. Structural analogies and differences between graphite oxide and C60 and C70 polymeric oxides (fullerene ozopolymers). Fuller Nanotub Car N 2003,11(1),113.
CrossRef - Anthemidis, A.N.; Zachariadis, G.A. ;Stratis, J.A., Talanta,2001, 54,935-943.
CrossRef - Zenedelovska, D.; Pavlovska, G.; Cundeva, K. ;Stafilov, T., Talanta2001 ,54,139-145.
CrossRef - Endo, M.; Suziki, K.; Abe, S., Anal. Chim.Acta ,1998,364,13-20.
CrossRef - Campderros, M.E.; Acosta, A. , J. Marchese, Talanta1998,47,19-26.
CrossRef - Narin, I.; Soylak, M. ;Elic, L.; Dogan, M., Talanta , 2000,52,1041-1049.
CrossRef - Moghimi A. Shabanzadeh M.,Journal of chemical Health Risks. 2012,2(2),1-7.
- A. Moghimi , Russian Journal of Physical Chemistry A,87(11) , 2013, 1851–1858.
- GoswamiA.,Singh A.K.,Venkataramani B.,Talanta. 2003,60, 1141-1153.
CrossRef - Moghimi A,.Aust J Basic &Appl Sci. 2012,6(3), 320-330.
- Moghimi A,. Chin J Chem. ,2007, 25(5),640-644.
CrossRef - Thurman, E.M.; Mills, M.S. ,Solid-Phase Extraction, Principles and Practice, 1998, Wiley, New York.
- Pawliszyn, J.; Solid-Phase Microextraction, Theory and Practice, 1997, Wiley-VCH, New York.
- Moghimi, A.;Yousefi Siahkalrodi, S., Journal of Chemical Health Risks ,2013,3(3), 01-12.
- Moghimi A., Afr J Pure Appl Chem. 2013, 7(2),79-90.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









