Electrochemical Removal of Hydrocortisone from Aqueous Environments using Aluminum Electrodes
Hashem Alaani , Shahir Hashem and François Karabet
, Shahir Hashem and François Karabet
Department of Chemistry, Faculty of Science, Damascus University, Damascus, Syria.
Corresponding Author E-mail: hashim.ani85@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340123
In this work, electrochemical removal of hydrocortisone, a steroid hormone, from water using aluminum electrodes was investigated. The effect of many experimental parameters such as current density, electrolysis time, initial concentration of hydrocortisone, initial pH, electrolyte type and concentration, and distance between electrodes were studied. Moreover, the sludge formed was characterized using high performance liquid chromatography (HPLC) and X-ray fluorescence (XRF). The electrical energy consumption was used to evaluate the economic feasibility of the electrochemical removal process. Results showed that the use of supporting electrolyte contains chloride ions has enhanced the removal efficiency due to the indirect electrooxidation by chlorine and hypochlorite generated electrochemically. Results demonstrated that the electrochemical treatment was simple, efficient, and cost effective method for removal of hydrocortisone from aqueous environments.
KEYWORDS:Electrochemical removal; Hydrocortisone; Aluminum electrodes; Energy consumption; Aqueous environments
Download this article as:| Copy the following to cite this article: Alaani H, Hashem S, Karabet F. Electrochemical Removal of Hydrocortisone from Aqueous Environments using Aluminum Electrodes. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Alaani H, Hashem S, Karabet F. Electrochemical Removal of Hydrocortisone from Aqueous Environments using Aluminum Electrodes. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42036 |
Introduction
Hydrocortisone (See Figure 1)1 is a steroid hormone produced by the adrenal cortex2. Its synthetic counterpart is used as a medication for treating asthma, inflammation, allergy, multiple sclerosis, and skin conditions3,4.
 |
Figure 1: Chemical Structure of hydrocortisone |
Steroid hormones are classified as Endocrine Disrupting Compounds (EDCs)5,6. EDCs may cause serious health effects in the organism5,6,7 because they can mimic or block the normal hormones functions 5,6,7. The exposure to these compounds may cause a decrease in male sperm count and increases in testicular, prostate, ovarian, and breast cancer6,8,9. Compared with all EDCs, steroid hormones have the greatest endocrine disrupting potency6,8.
Humans and animals continuously introduce steroid hormones into the environment6,9. Concentrations of these hormones have been detected in the fresh water bodies receiving effluent in North America, Europe, Japan, Brazil, and China6,9. The residues of the steroid hormones could find their way into the aquatic environment either by the excretions from humans and animals or by the remnants of the pharmaceutical industry.
During the past years, new methods have been developed for water purification. Electrochemical treatment is a simple, fast, efficient, and cost effective method for removal of pollutants from wastewater. Electrochemical treatment has been used for removal of various pollutants from aqueous solutions such as aromatic hydrocarbons10, leachate11, dyes12,13,14,15, and other pollutants16,17.
Generally, electrochemical treatment consists of many processes including electrooxidation, electrocoagulation, electroflocculation, and electroflotation. Electrooxidation of pollutants may be carried out directly (on the anode) or indirectly18. Indirect electrooxidation can occur, in the presence of high concentrations of chloride ions, by chlorine and hypochlorite generated due to the oxidation of chloride ions on the anode11. In the electrocoagulation process, coagulants are in-situ generated by electro-dissolution of a suitable sacrificial anode10 usually made of iron or aluminum14. Coagulants neutralize the electrostatic charges of pollutants present in wastewater to become destabilized and separated from the aqueous phase12,19. In the electroflocculation, metallic hydroxide flocs are formed when metal ions, released from the metal electrodes, combine with hydroxide ions. These flocs have large surface areas11,12,13 and can adsorb pollutants 19. Flocs are removed from the aqueous phase by sedimentation or electroflotation12 (flotation of flocculated particles and pollutants by hydrogen and oxygen bubbles generated electrochemically)10. The main reactions occurring when using aluminum electrodes are10,11,12,13,14,20:
At the anode:
Al → Al3+ + 3e− (1)
At the cathode:
3H2O + 3e− → 3/2 H2 (g) + 3OH− (2)
In the solution (at alkaline conditions):
Al3+ + 3OH− → Al(OH)3 (3)
Oxygen evolution reaction:
2H2O → O2 (g) + 4H+ + 4e− (4)
Chlorine and hypochlorite formation:
2Cl−→ Cl2 + 2e− (5)
Cl2 + H2O → HClO + H+ + Cl− (6)
HClO ↔ ClO− + H+ (7)
The aim of the present work is to evaluate the removal of hydrocortisone, a steroid hormone, from water by the electrochemical treatment using aluminum electrodes and to study the experimental parameters affecting the removal efficiency and the energy consumption.
Materials and Methods
Chemicals
Hydrocortisone sample was obtained from Symbiotica, Malaysia, and used as received without further purification. Sodium Chloride was purchased from Fisher Scientific, United Kingdom. Potassium Chloride was purchased from SHAMLAB, Syria. Methanol and Acetonitrile were of HPLC grade. Other reagents were of analytical grade. Water for injections21 (WFI), that has a conductivity less than 1 µs/cm, was used for the preparation of solutions.
Electrochemical experiments
The electrochemical system consisted of 1 L glass beaker as a reactor and a locally made direct current (DC) power supply unit (220 V input, 0–40 V output). An appropriate mixing was provided by a magnetic stirrer (Cole-Parmer, Malaysia). Aluminum rectangular plates with dimensions of (6.5 cm height, 3 cm width, and 0.35 mm thickness) were used as electrodes and placed vertically in the reactor. The total active surface area of the electrodes was 24 cm2. Figure 2 shows the schematic diagram of the electrochemical system.
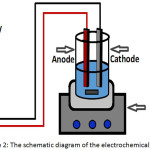 |
Figure 2: The schematic diagram of the electrochemical system |
Experiments were performed in batches19 at room temperature. In each experiment, 600 mL of hydrocortisone solution (with a specific concentration) was placed in the electrochemical reactor. Concentrations of NaCl or KCl were added to the hydrocortisone solution in order to investigate the effect of electrolyte type and concentration on the removal efficiency and energy consumption. Initial pH of the solution was adjusted with 0.1 M NaOH and 0.1 M HCl solutions using calibrated pH meter (Orion 320, USA). During the experiments, the hydrocortisone solution was continuously mixed by the magnetic stirrer at about 200 rpm.
After each electrochemical experiment, the electrodes were washed, with the aid of ultrasound, first with 0.1 M HCl solution and then with WFI. This process could overcome the electrode passivation17 that caused by the oxide film formed on the electrodes during the electrochemical treatment 17,19.
At the end of each run, the solution was kept without mixing for about 20 minutes to let the flocs settling down10. Then the supernatant was filtered using filter paper (MN713, Macherey-Nagel, Germany) and prepared for the analysis.
The concentration of hydrocortisone before and after the electrochemical treatment was determined using UV/VIS Spectrophotometer (CECIL, CE 7200, United Kingdom) at 248 nm as λmax. A calibration curve was plotted between absorbance and hydrocortisone concentration and the linearity was found to be satisfactory (R² = 0.9998). Additional dilutions of the hydrocortisone solution were performed when needed. Figure 3 shows the UV spectra of hydrocortisone before and after an electrochemical treatment.
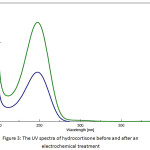 |
Figure 3: The UV spectra of hydrocortisone before and after an electrochemical treatment |
The removal efficiency (%) of the electrochemical treatment was estimated using the formula:
Removal % = [(C0-C)/C0] × 100 (8)
In which, C0 and C are the hydrocortisone concentrations (mg/L) before and after the electrochemical treatment, respectively.
The amount of hydrocortisone removed (µg) in each electrochemical experiment was determined using the formula:
Amount removed (µg) = Removal % × C0 × V × 1000 (9)
In which, Removal % is the removal efficiency (%), C0 is the initial concentration of hydrocortisone (mg/L) before the electrochemical treatment, and V is the volume (L) of the treated solution.
In order to evaluate the economic feasibility of the electrochemical removal process, the electrical energy consumption per mass (kWh g-1) was calculated using the following formula11,22,23:
Electrical energy consumption (kWh g-1) = [I.U.T]/[(C0-C)V] (10)
In which, I is the current (A), U is the voltage (V), T is the time (h), C0 and C are the hydrocortisone concentrations (mg/L) before and after the electrochemical treatment, respectively, and V is the volume (L) of the treated solution.
The electro-dissolution (g/L) of the aluminum anode was calculated according to Faraday’s law using the following formula10:
Anode electro-dissolution (g/L) = (I.t.M)/(z.F.V) (11)
In which10, I is the current (A), t is the electrolysis time (s), M (26.98 g mol-1) is the anode material atomic weight, z (zAl = 3) is the number of transferred electrons, F (96500 C mol-1) is Faraday’s constant, and V is the volume of treated solution (L).
In addition, the electrodes were weighed before and after the electrochemical experiments to determine the practical dissolution of the electrodes.
Further experiments were performed to evaluate the influence of several experimental parameters on the removal efficiency and energy consumption.
The sludge formed at the end of the electrochemical treatment was dried in the oven (Raven 2 Oven, LTE Scientific, United Kingdom) at 100o C for about 4 hours. After grinding the dried mass, a pale pink fine powder was obtained. This powder was analyzed using high performance liquid chromatography (HPLC) (LA Chrom ELITE, VWR Hitachi, Germany) and X-ray fluorescence (XRF). In addition, the loss on ignition (LOI) of the dried powder was determined using muffle furnace (Nabertherm, Germany).
Results and Discussion
Effect of Current Density
In order to investigate the influence of the current density on the electrochemical treatment, electrochemical experiments at current density ranged (from 15 to 35 mA/cm2) were performed. The other parameters were (electrolysis time: 120 min, initial concentration of hydrocortisone: 10 mg/L, electrolyte: 2.5 g/L NaCl, initial pH: 6.0, and the distance between electrodes: 4 cm). The results shown in (Table 1 and Figure 4) demonstrated that the removal efficiency increased with increasing current density. The increase of the current density may increase the formation of aluminum hydroxide flocs10,15,19 , the electro-generation of bubbles10, and the formation of chlorine and hypochlorite10. That could enhance the electroflocculation, electroflotation, and indirect electrooxidation.
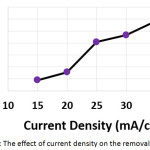 |
Figure 4: The effect of current density on the removal efficiency |
Although it is expected that the increase of the current density will increase the electrical energy consumption24, the highest energy consumption was at current density of 20 mA/cm2. That because the amount removed of hydrocortisone affects the value of electrical energy consumption per mass according to equation (10).
According to Faraday’s law, the electro-dissolution of the anode increases with increasing current density. That was clearly demonstrated in Table 1. However, the practical dissolution of the anode was found to be higher than the predicted values by the Faraday’s law22. This difference may be due to the chemical dissolution of the anode22,25.
The cathode dissolution may be explained by the chemical attack11,19,26 by hydroxide ions, generated during water reduction (equation 2), at high pH19:
2Al + 6H2O + 2OH− → 2Al(OH)4− + 3H2 (12)
Table 1: The effect of current density on the electrochemical treatment
|
Current density (mA/cm2) |
15 |
20 |
25 |
30 |
35 |
|
Removal % |
8.9% |
15.6% |
40.9% |
46.7% |
58.3% |
|
Electrical energy consumption (KWh g-1) |
14.56 |
14.97 |
8.26 |
10.23 |
10.71 |
|
Electro-dissolution of anode (predicted) (g) |
0.242 |
0.322 |
0.403 |
0.483 |
0.564 |
|
Practical dissolution of anode (g) |
0.308 |
0.401 |
0.522 |
0.586 |
0.710 |
|
Practical dissolution of cathode (g) |
0.132 |
0.137 |
0.211 |
0.252 |
0.310 |
Effect of Electrolysis time
To study the effect of electrolysis time on the electrochemical treatment, electrochemical experiments were performed with electrolysis times 30, 60, 90, 120, and 150 min. The other parameters were (current density: 30 mA/cm2, initial concentration of hydrocortisone: 10 mg/L, electrolyte: 2.5 g/L NaCl, initial pH: 6.0, and distance between electrodes: 4 cm). Results are summarized in (Table 2 and Figure 5).
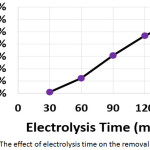 |
Figure 5: The effect of electrolysis time on the removal efficiency |
The removal efficiency increased with increasing electrolysis time due to the increase of generation of metal ions and flocs10,14,19. The dissolution of electrodes increased with increasing electrolysis time according to Faraday’s law10. The highest energy consumption was at electrolysis time of 30 min as a result of the extremely small amount of hydrocortisone removed after 30 min. While the lowest energy consumption was at electrolysis time of 120 min due to the huge amount of hydrocortisone removed after this time.
Table 2: The effect of electrolysis time on the electrochemical treatment
|
Electrolysis time (min) |
30 |
60 |
90 |
120 |
150 |
|
Removal % |
0.9% |
12.3% |
30.8% |
46.7% |
58.3% |
|
Electrical energy consumption (KWh g-1) |
146.00 |
19.61 |
12.39 |
10.23 |
10.34 |
|
Electro-dissolution of anode (predicted) (g) |
0.121 |
0.242 |
0.362 |
0.483 |
0.604 |
|
Practical dissolution of anode (g) |
0.187 |
0.294 |
0.451 |
0.586 |
0.734 |
Effect of Initial Concentration of Hydrocortisone
The influence of the initial concentration of hydrocortisone on the electrochemical treatment was studied with concentrations from 5 to 30 mg/L. The other parameters were (current density: 30 mA/cm2, electrolysis time: 120 min, electrolyte: 2.5 g/L NaCl, initial pH: 6.0, and distance between electrodes: 4 cm). Results shown in (Figures 6a, 6b, and 6c) demonstrated that the removal efficiency decreased slightly with increasing initial concentration. However, the amount of hydrocortisone removed increased with increasing initial concentration. This behavior was also observed in the removal of organic pollutants by photocatalysis27. The electrical energy consumption decreased with increasing initial concentration as a result of the increase of the amount removed of hydrocortisone, since the amount removed of hydrocortisone affects the value of electrical energy consumption per mass according to equation (10)11,22,23.
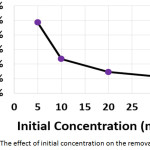 |
Figure 6a: The effect of initial concentration on the removal efficiency Click here to View figure |
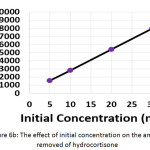 |
Figure 6b: The effect of initial concentration on the amount removed of hydrocortisone Click here to View figure |
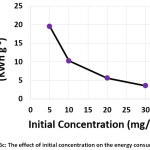 |
Figure 6c: The effect of initial concentration on the energy consumption Click here to View figure |
Effect of Distance Between Electrodes
The effect of the distance between electrodes on the electrochemical treatment was studied with distance ranged from 2 to 8 cm. The other parameters were (current density: 30 mA/cm2, electrolysis time: 120 min, electrolyte: 2.5 g/L NaCl, initial pH: 6.0, and initial concentration of hydrocortisone: 10 mg/L). It was found that the removal efficiency and the energy consumption increased with increasing the distance. These results correspond well with other published articles15,16,19. Results are summarized in Table 3.
Table 3: The effect of distance between electrodes on the electrochemical treatment
|
Distance between electrodes (cm) |
2 |
4 |
6 |
8 |
|
Removal % |
43.4% |
46.7% |
49.3% |
53.5% |
|
Electrical energy consumption (KWh g-1) |
7.69 |
10.23 |
12.90 |
14.09 |
Effect of Initial pH
To evaluate the effect of the initial pH of the treated solution on the electrochemical treatment, pH values have been varied from 4 to 10. The other parameters were (current density: 30 mA/cm2, electrolysis time: 120 min, electrolyte: 2.5 g/L NaCl, distance between electrodes: 4 cm, and initial concentration of hydrocortisone: 10 mg/L). Results shown in Figure 7 demonstrated that the optimal pH value was 6. Although the literature survey revealed that the initial pH was one of the important factors affecting the electrochemical treatment19,28,29, our findings showed that the initial pH has no significant impact on the removal efficiency.
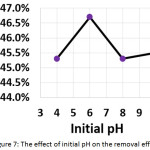 |
Figure 7: The effect of initial pH on the removal efficiency Click here to View figure |
Effect of Electrolyte Type and Concentration
To investigate this factor, NaCL (at a concentration of 1.5, 2, and 2.5 g/L) and KCl (at a concentration of 2 g/L) were used as supporting electrolytes. The other parameters were (current density: 30 mA/cm2, electrolysis time: 120 min, distance between electrodes: 4 cm, initial pH: 6.0, and initial concentration of hydrocortisone: 10 mg/L). Results are summarized in Figures 8a, 8b, 8c, and 8d. The optimal NaCl concentration was 2g/L that achieved higher removal efficiency and less electrical energy consumption. Comparing between NaCl and KCl at a constant concentration of 2 g/L, It was found that NaCl was the most favorable electrolyte.
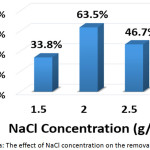 |
Figure 8a: The effect of NaCl concentration on the removal efficiency Click here to View figure |
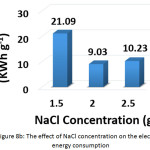 |
Figure 8b: The effect of NaCl concentration on the electrical energy consumption Click here to View figure |
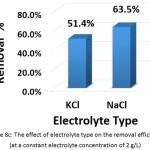 |
Figure 8c: The effect of electrolyte type on the removal efficiency (at a constant electrolyte concentration of 2 g/L) Click here to View figure |
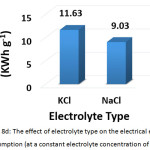 |
Figure 8d: The effect of electrolyte type on the electrical energy consumption (at a constant electrolyte concentration of 2 g/L) Click here to View figure |
Generally, the presence of the electrolyte increases the ionic strength30 and the conductivity. That causes a reduction of the cell voltage at constant current density19,31 and minimizes the electrical energy consumption19.
The increase of the removal efficiency in the presence of electrolyte contains chloride ions could be caused by the indirect electrooxidation by chlorine and hypochlorite generated electrochemically32 (reactions 5, 6, and 7).
Characterization of the Sludge
After drying and grinding the sludge, a pale pink fine powder was obtained. The loss on ignition (LOI) of the dried powder, determined at 550o C until constant weight, was found to be 26.7%. This value equates to the organic matter and volatile solids content33.
In addition to Al2O3, the detected by-products formed after electrochemical treatment33 were elements like Fe, Ni, Mn, and Zn as XRF analysis showed (Table 4).
In order to determine the residual amount of hydrocortisone in the sludge, a portion of the dried powder was transferred to a volumetric flask containing ethanol (a suitable solvent for hydrocortisone extraction), and shaken by mechanical means and with the aid of ultrasound for about one hour to extract the hydrocortisone. Then the solution was filtered and analyzed using HPLC. Table 5 shows the chromatographic conditions of the analytical procedure. Figures 9a, 9b, and 9c show the representative chromatograms of the hydrocortisone standard solution, the solution of the studied sludge, and the solvent used in the extraction process (Ethanol), respectively. It was found that no traces of hydrocortisone were detected in the dried sludge. That may be explained by the destruction of hydrocortisone by the oxidants (Cl2, ClO−) generated during the electrochemical treatment. The chromatograms also showed that the peaks obtained at the retention times (from 1.6 to 2.1 min) were ascribed to the ethanol. The recovery of the extraction process was determined by adding a known quantity of hydrocortisone to the sludge and then extract this quantity. The recovery (%) of the extraction process was found to be 99.4%.
Table 4: XRF results of the dried sludge*
|
Element |
Concentration (µg/g) |
|
Cr |
31.1 ± 6.6 |
|
Mn |
821 ± 68 |
|
Fe |
525 ± 32 |
|
Ni |
4.81 ± 0.96 |
|
Cu |
97.4 ± 5.8 |
|
Zn |
15.6 ± 1.3 |
|
Ga |
6.74 ± 0.70 |
|
Pb |
1.97 ± 0.30 |
* Results are valid for sample of Al2O3 matrix.
Table 5: The chromatographic conditions of the analytical procedure
|
Column |
C18 (150 mm, 4.6 mm i.d., 5 μm) |
|
Mobile phase |
50% water, 25% Methanol, 25% Acetonitrile |
|
Flow rate |
1 mL/minute |
|
Column temperature |
40oC |
|
Detection wavelength |
248 nm |
|
Injection volume |
10 μL |
|
Standard solution |
Hydrocortisone, 0.04 mg/mL (in ethanol) |
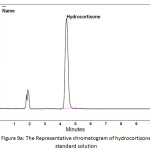 |
Figure 9a: The Representative chromatogram of hydrocortisone standard solution Click here to View figure |
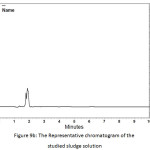 |
Figure 9b: The Representative chromatogram of the studied sludge solution Click here to View figure |
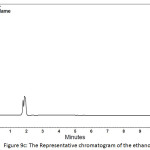 |
Figure 9c: The Representative chromatogram of the ethanol Click here to View figure |
Coclusion
These findings indicated that the electrochemical treatment using aluminum electrodes was an appropriate choice for removal of hydrocortisone from water. The optimal values of experimental parameters that achieved the suitable electrical energy consumption, removal efficiency, and electrode consumption were: current density: 30 mA/cm2, electrolysis time: 120 min, distance between electrodes: 4 cm, initial pH: 6.0, electrolyte: 2 g/L NaCl, and initial concentration of hydrocortisone: 30 mg/L. Indirect electrooxidation, by chlorine and hypochlorite, played a major role in the destruction of hydrocortisone.
Acknowledgment
The authors would like to thank the quality control team in DIAMOND PHARMA for pharmaceutical industries, Syria, especially Ms. Douaa Almoukdad for the support.
The authors would also like to thank the central laboratory in Damascus University, Faculty of Science, Department of Chemistry, for providing the XRF analysis.
References
- British Pharmacopoeia 2013, Volume I &II, Monographs: Medicinal and Pharmaceutical Substances, Hydrocortisone. London: The Stationery Office.
- Sweetman, S. C.; Pharm, B.; Pharm, S. F. R. Martindale: The Complete Drug Reference. 36th ed. London: Pharmaceutical Press, 2009, page: 1536.
- Hydrocortisone. Drugs.com. Available from: https://www.drugs.com/cdi/hydrocortisone.html. [Accessed on 20 August 2017].
- Hydrocortisone. Pubchem. Open Chemistry Database. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/hydrocortisone. [Accessed on 20 August 2017].
- AlAani, H.; Hashem, S.; Karabet, F. Photocatalytic (UV-A/TiO2) degradation and photolytic (UV-A) degradation of steroid hormones: norethisterone and danazol in aqueous medium. International Journal of ChemTech Research. 2014, 6(7), 3725-3732.
- AlAani, H.; Hashem, S.; Karabet, F. Photocatalytic (UV-A/TiO2) and photolytic (UV-A) degradation of steroid hormones: Ethinyl Estradiol, Levonorgestrel, and Progesterone. International Journal of ChemTech Research. 2017, 10(2), 1061-1070.
- Wen, H. Removal of estrone from water with adsorption and UV photolysis. M.Sc. thesis, Worcester Polytechnic Institute, Worcester, Massachusetts, USA. 2006.
- Nghiem, L. D.; Schäfer, A. I.; Elimelech, M. Removal of natural hormones by nanofiltration membranes: measurement, modeling, and mechanisms. Environmental science & technology. 2004, 38(6), 1888-1896.
CrossRef - Nghiem, L. D.; Manis, A.; Soldenhoff, K.; Schäfer, A. I. Estrogenic hormone removal from wastewater using NF/RO membranes. Journal of Membrane Science. . 2004, 242(1), 37-45.
CrossRef - AlSalka, Y.; Karabet, F.; Hashem, S. Evaluation of electrochemical processes for the removal of several target aromatic hydrocarbons from petroleum contaminated water. Journal of Environmental Monitoring. 2011, 13(3), 605-613.
CrossRef - Li, J.; Yang, Z. H.; Xu, H. Y.; Song, P. P.; Huang, J.; Xu, R.; Zhang, Y. J.; Zhou, Y. Electrochemical treatment of mature landfill leachate using Ti/RuO2–IrO2 and Al electrode: optimization and mechanism. RSC Advances. 2016, 6(53), 47509-47519.
CrossRef - Asghari, A.; Kamalabadi, M.; Farzinia, H. Electrochemical removal of methylene blue from aqueous solutions using taguchi experimental design. Chemical and Biochemical Engineering Quarterly. 2012, 26(2), 145-154.
- De Carvalho, H. P.; Huang, J.; Zhao, M.; Liu, G.; Dong, L.; Liu, X. Improvement of Methylene Blue removal by electrocoagulation/banana peel adsorption coupling in a batch system. Alexandria Engineering Journal. 2015, 54(3), 777-786.
CrossRef - Mahmoud, M. S.; Farah, J. Y.; Farrag, T. E. Enhanced removal of Methylene Blue by electrocoagulation using iron electrodes. Egyptian Journal of Petroleum. 2013, 22(1), 211-216.
CrossRef - Alizadeh, M.; Ghahramani, E.; Zarrabi, M.; Hashemi, S. Efficient De-colorization of Methylene Blue by Electro-coagulation Method: Comparison of Iron and Aluminum Electrode. Iranian Journal of Chemistry and Chemical Engineering. 2015, 34(1), 39-47.
- Pinedo-Hernandez, J.; Nunez, Y.; Sanchez, I.; Marrugo-Negrete, J. Treatment of Meat Industry Wastewater Using Electrochemical Treatment Method. Portugaliae Electrochimica Acta. 2015, 33(4), 223-230.
CrossRef - Bazrafshan, E.; Biglari, H.; Mahvi, A. H. Humic acid removal from aqueous environments by electrocoagulation process using iron electrodes. Journal of Chemistry. 2012, 9(4), 2453-2461.
CrossRef - Martínez-Huitle, C. A. Direct and indirect electrochemical oxidation of organic pollutants. Ph.D. thesis, University of Ferrara, Italy.2004.
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A. H. Heavy metals removal from aqueous environments by electrocoagulation process–a systematic review. Journal of environmental health science and engineering. 2015, 13(1), 74.
CrossRef - Klamklang, S. Restaurant wastewater treatment by electrochemical oxidation in continuous process. Ph.D. thesis, Institut National Polytechnique de Toulouse. 2007.
- British Pharmacopoeia 2013, Volume I &II, Monographs: Medicinal and Pharmaceutical Substances, Water for Injections. London: The Stationery Office.
- Secula, M. S.; Creţescu, I.; Petrescu, S. Electrocoagulation treatment of sulfide wastewater in a batch reactor: effect of electrode material on electrical operating costs. Environmental Engineering & Management Journal. 2012, 11(8), 1485-1491.
CrossRef - Apaydin, Ö.; Kurt, U.; Gonullu, M. T. An investigation on the treatment of tannery wastewater by electrocoagulation. Global NEST Journal. 2009, 11(4), 546-555.
- Can, B. Z.; Boncukcuoglu, R.; Yilmaz, A. E.; Fil, B. A. Effect of some operational parameters on the arsenic removal by electrocoagulation using iron electrodes. Journal of Environmental Health Science and Engineering. 2014, 12(1), 95.
CrossRef - Cañizares, P.; Martínez, F.; Jiménez, C.; Sáez, C.; Rodrigo, M. A. Technical and economic comparison of conventional and electrochemical coagulation processes. Journal of chemical technology and biotechnology. 2009, 84(5), 702-710.
CrossRef - Picard, T.; Cathalifaud-Feuillade, G.; Mazet, M.; Vandensteendam, C. Cathodic dissolution in the electrocoagulation process using aluminium electrodes. Journal of Environmental Monitoring. 2000, 2(1), 77-80.
CrossRef - Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N. P.; Mantzavinos, D.; Fatta-Kassinos, D. Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis. Chemical Engineering Journal. 2010, 161(1), 53-59.
CrossRef - Gengec, E.; Kobya, M.; Demirbas, E.; Akyol, A.; Oktor, K. Optimization of baker’s yeast wastewater using response surface methodology by electrocoagulation. Desalination. 2012, 286, 200-209.
CrossRef - Thirugnanasambandham, K.; Sivakumar, V.; Prakash Maran, J. Evaluation of an electrocoagulation process for the treatment of bagasse‐based pulp and paper industry wastewater. Environmental Progress & Sustainable Energy. 2015, 34(2), 411-419.
CrossRef - Khandegar, V.; Saroha, A. K. Electrochemical treatment of textile effluent containing Acid Red 131 dye. Journal of Hazardous, Toxic, and Radioactive Waste. 2013, 18(1), 38-44.
CrossRef - Merzouk, B.; Madani, K.; Sekki, A. Using electrocoagulation–electroflotation technology to treat synthetic solution and textile wastewater, two case studies. Desalination. 2010, 250(2), 573-577.
CrossRef - Mahmoud, S. S.; Ahmed, M. M. Removal of surfactants in wastewater by electrocoagulation method using iron electrodes. Physical Sciences Research International. 2014, 2(2), 28-34.
- Hoseinzadeh, E.; Rezaee, A. Electrochemical degradation of RB19 dye using low-frequency alternating current: effect of a square wave. RSC Advances. 2015, 5(117), 96918-96926.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









